UVC-Assisted Tertiary Treatments for the Removal of Pollutants of Emerging Concern in Real WWTP Matrices
Abstract
:1. Introduction
2. Experimental Part
2.1. Reagents and Chemicals
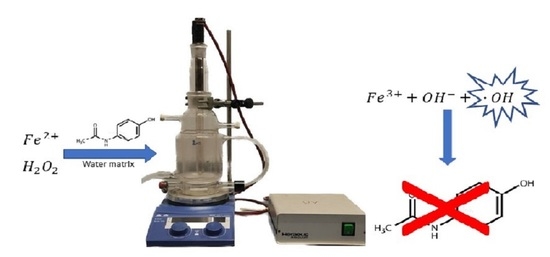
2.2. Reactions
2.3. Chemical Analysis
2.4. Statistical Treatment of Data
3. Results and Discussion
3.1. Photolysis of Acetaminophen
3.2. Photo-Fenton Process
3.3. Combination of the Photochemical Processes with Coagulation Flocculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN-Water Analytical Brief Water-Use Efficiency. 2021. Available online: https://www.unwater.org/publications/un-water-analytical-brief-water-use-efficiency. (accessed on 4 October 2022).
- Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741 (accessed on 24 November 2022).
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.G.; Maqbool, F.; Hu, Y. Removal of antibiotics pollutants in wastewater by UV-based advanced oxidation processes: Influence of water matrix components, processes optimization and application: A review. J. Water Process Eng. 2022, 45, 102496. [Google Scholar] [CrossRef]
- Kumar, S.; Ghosh, N.C.; Kazmi, A.A. Ballasted sand flocculation for water, wastewater and CSO treatment. Environ. Technol. Rev. 2016, 5, 57–67. [Google Scholar] [CrossRef]
- Rizzo, L. Addressing main challenges in the tertiary treatment of urban wastewater: Are homogeneous photodriven AOPs the answer? Environ. Sci. Water Res. Technol. 2022, 8, 2145. [Google Scholar] [CrossRef]
- de Boer, S.; Gonzalez-Rodríguez, J.J.; Conde, J.J.; Moreira, M.T. Benchmarking tertiary water treatments for the removal of micropollutants and pathogens based on operational and sustainability criteria. J. Water Process Eng. 2022, 46, 102587. [Google Scholar] [CrossRef]
- Nazir, R.; Khan, M.; Rehman, R.U.; Shujah, S.; Khan, M.; Ullah, M.; Zada, A.; Mahmood, N.; Ahmad, I. Adsorption of selected azo dyes from an aqueous solution by activated carbon derived from Monotheca buxifolia waste seeds. Soil Water Res. 2020, 15, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Nazir, R.; Khan, M.; Khan, W.; Shah, M.; Afridi, S.G.; Zada, A. The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells. Soil Water Res. 2020, 15, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Clancy, J.L.; Hargy, T.M.; Marshall, M.M.; Dyksen, J.E. UV light inactivation of Cryptosporidium oocysts. J. Am. Water Works Assoc. 1998, 90, 92–102. [Google Scholar] [CrossRef]
- Zhang, X.; Kamali, M.; Zhang, S.; Yu, X.; Appels, L.; Cabooter, D.; Dewil, R. Photo-assisted (waste)water treatment technologies —A scientometric-based critical review. Desalination 2022, 538, 115905. [Google Scholar] [CrossRef]
- Solomou, N.; Minella, M.; Vione, D.; Psillakis, E. UVC-induced degradation of cilastatin in natural water and treated wastewater. Chemosphere 2021, 280, 130668. [Google Scholar] [CrossRef]
- Pozdnyakov, I.P.; Snytnikova, O.A.; Yanshole, V.V.; Fedunov, R.G.; Grivin, V.P.; Plyusnin, V.F. Direct UV photodegradation of herbicide triclopyr in aqueous solutions: A mechanistic study. Chemosphere 2022, 293, 133573. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Z.; Zhou, B.; Chen, H. Yuan. Impact of water matrices on oxidation effects and mechanisms of pharmaceuticals by ultraviolet-based advanced oxidation technologies: A review. Sci. Total Environ. 2022, 844, 157162. [Google Scholar] [CrossRef] [PubMed]
- Wols, B.A. CFD in drinking water treatment. 4TU. ResearchData. Collection. Available online: https://data.4tu.nl/collections/CFD_in_drinking_water_treatment/5065466 (accessed on 24 February 2023).
- Lopez, J.L.; Einschlag, F.S.; Gonzalez, M.C.; Capparelli, A.L.; Oliveros, E.; Hashem, T.M.; Braun, A.M. Hydroxyl radical initiated photodegradation of 4-chloro-3,5-dinitrobenzoic acid in aqueous solution. J. Photochem. Photobiol. A Chem. 2000, 137, 177–184. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simón, P.J.; Lardín, C.; Martínez, M.A.; Vicente, J.A.; González, R.; Llosá, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Bernabeu, A.; Palacios, S.; Vicente, R.; Vercher, R.; Malato, S.; Arques, A.; Amat, A.M. Solar photo-Fenton at mild conditions to treat a mixture of six emerging pollutants. Chem. Eng. J. 2012, 198–199, 65–72. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A. Modified photo-Fenton for degradation of emerging contaminants in municipal wastewater effluents. Catal. Today 2011, 161, 241–246. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Amat, A.M.; Arques, A. Strategies to Drive Photo-Fenton Process at Mild Conditions for the Removal of Xenobiotics from Aqueous Systems. Curr. Org. Chem. 2017, 21, 1074–1083. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Vallés, I.; García-Negueroles, P.; Santos-Juanes, L.; Arques, A. Enhancement of iron-based photo-driven processes by the presence of catechol moieties. Catalysts 2021, 11, 372. [Google Scholar] [CrossRef]
- García-Ballesteros, S.; García-Negueroles, P.; Amat, A.M.; Arques, A. Humic-like substances as auxiliaries to enhance advanced oxidation processes. ACS Omega 2022, 7, 3151–3157. [Google Scholar] [CrossRef]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.C.; Teixeira, S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Burns, E.E.; Carter, L.J.; Kolpin, D.W.; Thomas-Oates, J.; Boxall, A.B.; Boxall, A. Temporal and spatial variation in pharmaceutical concentrations in an urban river system. Water Res. 2018, 137, 72–85. [Google Scholar] [CrossRef]
- Martínez Bueno, M.J.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Santos dos, W.N.L.; Quintella, C.M.; Neto b, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter–filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photoche. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef]
- Scaiano, J.C.T. Photochemistry Essentials. Am. Chem. Soc. Focus 2022, 25. [Google Scholar] [CrossRef]
- García-Ballesteros, S.; Mora, M.; Vicente, R.; Sabater, C.; Castillo, M.A.; Arques, A.; Amat, A.M. Gaining further insight into photo-Fenton treatment of phenolic compounds commonly found in food processing industry. Chem. Eng. J. 2016, 288, 126–136. [Google Scholar] [CrossRef]
- Yang, L.; Hur, J.; Zhuang, W. Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: A review Environ. Sci. Pollut. Res. 2015, 22, 6500–6510. [Google Scholar] [CrossRef]
- Sciscenko, I.; Arques, A.; Micó, P.; Mora, M.; García-Ballesteros, S. Emerging applications of EEM-PARAFAC for water treatment: A concise review. Chem. Eng. J. Adv. 2022, 10, 100286. [Google Scholar] [CrossRef]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205–206, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef] [PubMed]










| Experiment | [H2O2] (mM) | Flow (L·h−1) | Degradation (%) |
|---|---|---|---|
| 1 | 1.1 | 2.9 | 58.6% |
| 2 | 2.1 | 2.9 | 76.8% |
| 3 | 1.6 | 3.9 | 71.4% |
| 4 | 0.1 | 2.9 | 24.7% |
| 5 | 0.6 | 1.8 | 66.3% |
| 6 | 1.6 | 1.8 | 80.7% |
| 7 | 0.6 | 3.9 | 44.4% |
| 8 | 1.1 | 2.9 | 58.4% |
| 9 | 1.07 | 2.9 | 57.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Timoner, R.; Mora, M.; Zuriaga, E.; Climent, J.; Santos-Juanes, L.; Amat, A.M.; Arques, A. UVC-Assisted Tertiary Treatments for the Removal of Pollutants of Emerging Concern in Real WWTP Matrices. Water 2023, 15, 882. https://doi.org/10.3390/w15050882
López-Timoner R, Mora M, Zuriaga E, Climent J, Santos-Juanes L, Amat AM, Arques A. UVC-Assisted Tertiary Treatments for the Removal of Pollutants of Emerging Concern in Real WWTP Matrices. Water. 2023; 15(5):882. https://doi.org/10.3390/w15050882
Chicago/Turabian StyleLópez-Timoner, Rubén, Margarita Mora, Elena Zuriaga, Javier Climent, Lucas Santos-Juanes, Ana M. Amat, and Antonio Arques. 2023. "UVC-Assisted Tertiary Treatments for the Removal of Pollutants of Emerging Concern in Real WWTP Matrices" Water 15, no. 5: 882. https://doi.org/10.3390/w15050882
APA StyleLópez-Timoner, R., Mora, M., Zuriaga, E., Climent, J., Santos-Juanes, L., Amat, A. M., & Arques, A. (2023). UVC-Assisted Tertiary Treatments for the Removal of Pollutants of Emerging Concern in Real WWTP Matrices. Water, 15(5), 882. https://doi.org/10.3390/w15050882