Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
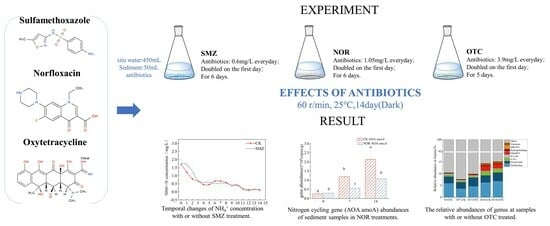
2.2. Batch Treatment
2.3. Determination of the Water Quality Index
2.4. Determination of Functional Genes of the Nitrogen Cycle
2.5. High-Throughput Sequencing
2.6. Data Analysis
3. Results
3.1. Changes in Various Nitrogen Compounds in Water
3.2. Nitrogen-Cycling Gene Abundance
3.3. Composition of Microbiome Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.H.; Zhang, G.; Zou, S.C.; Li, X.D.; Liu, Y.C. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Fahrenfeld, N.; Knowlton, K.; Krometis, L.A.; Hession, W.C.; Xia, K.; Lipscomb, E.; Libuit, K.; Green, B.L.; Pruden, A. Effect of manure application on abundance of antibiotic resistance genes and their attenuation rates in soil: Field-scale mass balance approach. Environ. Sci. Technol. 2014, 48, 2643–2650. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Wen, L.-L.; Song, J.-M.; Li, X.-G.; Ma, J.; Dai, J.-J.; Yuan, H.-M.; Duan, L.-Q.; Wang, Q.-D. Environmental pollution of fluoroquinolones and its relationship with nitrogen cycling mediated by microorganisms. Ying Yong Sheng Tai Xue Bao 2023, 34, 3114–3126. [Google Scholar]
- Wang, M.J.; Huang, Z.Q.; Zhang, B.B.; Shi, X.Z. Soil nitrification and denitrification in Cunninghamia lanceolata plantations with different stand ages. Ying Yong Sheng Tai Xue Bao 2023, 34, 18–24. [Google Scholar]
- Yin, G.; Hou, L.; Liu, M.; Zheng, Y.; Li, X.; Lin, X.; Gao, J.; Jiang, X. Effects of thiamphenicol on nitrate reduction and N2O release in estuarine and coastal sediments. Environ. Pollut. 2016, 214, 265–272. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.C.; Dou, Z.X. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Lu, C.; Guo, J.; Li, H.; Song, Y.; Han, Y.; Hou, Y. Effect and ameliorative mechanisms of polyoxometalates on the denitrification under sulfonamide antibiotics stress. Bioresour. Technol. 2020, 305, 123073. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Zou, Y.; Lin, M.; Xiong, W.; Wang, M.; Zhang, J.; Wang, M.; Sun, Y. Metagenomic insights into the effect of oxytetracycline on microbial structures, functions and functional genes in sediment denitrification. Ecotox Environ. Safe 2018, 161, 85–91. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Marques, C.N.; Morozov, A.; Planzos, P.; Zelaya, H.M. The fatty acid signaling molecule cis-2-decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial-susceptible state. Appl. Environ. Microb. 2014, 80, 6976–6991. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, R.; Liang, Y.-T.; Shu, X.-M.; Xu, C.-C.; Chen, L.-J. Influence of Ciprofloxacin on the Microbial Community and Antibiotics Resistance Genes in a Membrane Bioreactor. Environ. Sci. 2018, 39, 1333–1341. [Google Scholar]
- Li, S.; Ma, B.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Gao, M. Effect of norfloxacin on performance, microbial enzymatic activity and microbial community of a sequencing batch reactor. Environ. Technol. Inno. 2020, 18, 100726. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Piotrowska-Seget, Z. Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Sci. Total Environ. 2019, 650, 2951–2961. [Google Scholar] [CrossRef]
- Xi, X.; Wang, M.; Chen, Y.; Yu, S.; Hong, Y.; Ma, J.; Wu, Q.; Lin, Q.; Xu, X. Adaption of the microbial community to continuous exposures of multiple residual antibiotics in sediments from a salt-water aquacultural farm. J. Hazard. Mater. 2015, 290, 96–105. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Chen, Y.; Xu, J.; Yang, B.; Jiang, J. Ecological effects of antibiotics on aquaculture ecosystems based on microbial community in sediments. Ocean. Coast. Manag. 2022, 224, 106173. [Google Scholar] [CrossRef]
- Tong, X.N.; Wang, X.Z.; He, X.J.; Wang, Z.; Li, W.X. Effects of antibiotics on microbial community structure and microbial functions in constructed wetlands treated with artificial root exudates. Environ. Sci. Process. Impacts 2020, 22, 217–226. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.-P.; Fu, J.; Zhou, Z.-Q.; Zhang, N.; Guo, L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fert. Soils 2014, 50, 939–947. [Google Scholar] [CrossRef]
- Trifonova, T.; Kosmacheva, A.G.; Chesnokova, S.M. Effect of antibiotics on the cellulolytic and nitrification activity of gray forest soil. South. Russ. Ecol. Dev. 2020, 15, 52–62. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Ollivier, J.; Schacht, D.; Kindler, R.; Groeneweg, J.; Engel, M.; Wilke, B.-M.; Kleineidam, K.; Schloter, M. Effects of repeated application of sulfadiazine-contaminated pig manure on the abundance and diversity of ammonia and nitrite oxidizers in the root-rhizosphere complex of pasture plants under field conditions. Front. Microbiol. 2013, 4, 22. [Google Scholar] [CrossRef]
- Gentle, B.S.; Ellis, P.S.; Grace, M.R.; McKelvie, I.D. Flow analysis methods for the direct ultra-violet spectrophotometric measurement of nitrate and total nitrogen in freshwaters. Anal. Chim. Acta 2011, 704, 116–122. [Google Scholar] [CrossRef]
- Wang, R.; Deng, L.; Fan, X.; Li, K.; Lu, H.; Li, W. Removal of heavy metal ion cobalt (II) from wastewater via adsorption method using microcrystalline cellulose-magnesium hydroxide. Int. J. Biol. Macromol. 2021, 189, 607–617. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Wen, G.Q.; Li, J.; Liu, X.H.; Zhang, Y.S.; Wen, S.S. Extraction of total DNA and optimization of the RAPD reaction system in Dioscorea opposita Thunb. Genet. Mol. Res. 2014, 13, 1339–1347. [Google Scholar] [CrossRef]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Enwall, K.; Philippot, L.; Hallin, S. Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl. Environ. Microb. 2005, 71, 8335–8343. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Song, C.; Dong, X.Y.; Fan, L.M.; Zhang, C.; Qiu, L.P.; Chen, J.R. Effect of Sulfamethoxazole on Water Bacterial Community Structure in Tilapia (Oreochromis niloticus) Culture Pond. Environ. Sci. Technol. 2019, 42, 1–11. (In Chinese) [Google Scholar]
- Wang, Y.N.; Guo, X.C.; Lu, S.Y.; Liu, X.H.; Wang, X.H. Review of nitrogen removal in low-polluted water by constructed wetlands: Performance, mechanism, and influencing factors. J. Agric. Resour. Environ. 2021, 38, 722–734. (In Chinese) [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, J.; Wan, L.; Sun, S.; MacIsaac, H.J.; Drouillard, K.G.; Chang, X. Norfloxacin pollution alters species composition and stability of plankton communities. J. Hazard. Mater. 2020, 385, 121625. [Google Scholar] [CrossRef]
- Chen, L.; Huang, F.; Zhang, C.; Zhang, J.; Liu, F.; Guan, X. Effects of norfloxacin on nitrate reduction and dynamic denitrifying enzymes activities in groundwater. Environ. Pollut. 2021, 273, 116492. [Google Scholar] [CrossRef]
- Wei, D.B. Single and Joint Toxicity of Oxytetracycline, Enrofloxacin, Sulfadimidine and Cu on Soil Microorganism. Master’s Thesis, Shandong Agricultural University, Taian, China, 2014. (In Chinese). [Google Scholar]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele-Bruhn, S.; Sharma, S.; Wilke, B.; Matthies, M.; Smalla, K.; et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009, 11, 446–456. [Google Scholar] [CrossRef]
- Coombs, K.; Rodriguez-Quijada, C.; Clevenger, J.O.; Sauer-Budge, A.F. Current Understanding of Potential Linkages between Biocide Tolerance and Antibiotic Cross-Resistance. Microorganisms 2023, 11, 2000. [Google Scholar] [CrossRef]
- Orellana, L.H.; Rodriguez-R, L.M.; Higgins, S.; Chee-Sanford, J.C.; Sanford, R.A.; Ritalahti, K.M.; Löffler, F.E.; Konstantinidis, K.T. Detecting nitrous oxide reductase (NosZ) genes in soil metagenomes: Method development and implications for the nitrogen cycle. mBio 2014, 5, e01193-14. [Google Scholar] [CrossRef]
- Li, P.; Chen, C.Z.; Zhao, X.L.; Liu, L.; Li, Z.H. Metagenomics analysis reveals the effects of norfloxacin on the gut microbiota of juvenile common carp (Cyprinus carpio). Chemosphere 2023, 325, 138389. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, J.; Niu, L.; Zhang, W.; Cai, W.; Zhu, X. Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. Environ. Sci. Pollut. R. 2017, 24, 19795–19806. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chen, D.X.; Liao, M.J.; Wang, S.L.; Li, Z. Spatiotemporal distribution of aerobic ammonia-oxidizing microorganisms in sediments of Lake Donghu, Wuhan. China Environ. Sci. 2021, 41, 1917–1924. (In Chinese) [Google Scholar]
- Abd Aziz, A.; Lee, K.; Park, B.; Park, H.; Park, K.; Choi, I.G.; Chang, I.S. Comparative study of the airborne microbial communities and their functional composition in fine particulate matter (PM 2.5 ) under non-extreme and extreme PM 2.5 conditions. Atmos. Environ. 2018, 194, 82–92. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Barboza, A.D.M.; Pereira, A.B.; Triplett, E.W.; Schaefer, C.E.G.R.; Camargo, F.A.d.O.; Roesch, L.F.W. Distribution and interaction patterns of bacterial communities in an ornithogenic soil of Seymour Island, Antarctica. Microb. Ecol. 2015, 69, 684–694. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Han, R.; Luo, W.; Zheng, L. Migration of antibiotic resistance genes and evolution of flora structure in the Xenopus tropicalis intestinal tract with combined exposure to roxithromycin and oxytetracycline. Sci. Total Environ. 2022, 820, 153176. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, C.; Han, R.; Li, S.; Peng, H.; Zhou, X.; Huang, L.; Xu, Y. Oxytetracycline and heavy metals promote the migration of resistance genes in the intestinal microbiome by plasmid transfer. ISME J. 2023, 17, 2003–2013. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Zhu, K.; Zhang, H. Influence of oxytetracycline on the structure and activity of microbial community in wheat rhizosphere soil. J. Environ. Sci. -China 2009, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]









| Functional Gene | Name of Primers | Primer Sequence (5′ to 3′) | Ref. |
|---|---|---|---|
| AOA amoA | CrenamoA23f | ATGGTCTGGCTWAGACG | [31] |
| CrenamoA616r | GCCATCCABCKRTANGTCCA | ||
| AOB amoA | nxrB-F | TACATGTGGTGGAACA | [32] |
| nxrB-R | CGGTTCTGGTCRATCA | ||
| nxrB | amoA-1F | GGGGTTTCTACTGGTGGT | [33] |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | ||
| nosZ | nosZ-F | CGYTGTTCMTCGACAGCCAG | [34] |
| nosZ-1622R | CGSACCTTSTTGCCSTYGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; He, H.; Ding, J.; Zhang, Z.; Leng, Y.; Liao, M.; Xiong, W. Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water 2024, 16, 1256. https://doi.org/10.3390/w16091256
Li Z, He H, Ding J, Zhang Z, Leng Y, Liao M, Xiong W. Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water. 2024; 16(9):1256. https://doi.org/10.3390/w16091256
Chicago/Turabian StyleLi, Zhu, Huan He, Jianhe Ding, Zhizhong Zhang, Yifei Leng, Mingjun Liao, and Wen Xiong. 2024. "Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water" Water 16, no. 9: 1256. https://doi.org/10.3390/w16091256
APA StyleLi, Z., He, H., Ding, J., Zhang, Z., Leng, Y., Liao, M., & Xiong, W. (2024). Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water, 16(9), 1256. https://doi.org/10.3390/w16091256