Evaluating the Measurement of Activated Sludge Foam Potential
Abstract
:1. Introduction
1.1. Common Methods Used to Determine Foam Stability and ‘Foamability’
1.2. Assessing Severity of Activated Sludge Foaming and Use of Foam Potential
| Summary of method | Description of key apparatus | Ref. |
|---|---|---|
| 500 mL of activated sludge at specific mixed liquor suspended solids (MLSS) of 3,340 mg SS/L was aerated in a graduated cylinder and aerated with a flow-rate of 2 L min−1 through sintered sand diffuser for 60 seconds. Foam height was recorded every 15secs throughout this period. | Graduated cylinder with sintered sand diffuser. | [53] |
| 20 mL of sample (filament cell culture) was placed in a glass column with sintered glass disc through which samples were aerated with industrial grade air at 100 mL min−1 for 1 min. Test used to measure foam stability assessed by the time taken after the cessation of air. | Specially blown cylinder of diameter 21 mm and length 210 mm. | [6] |
| 250 mL of activated sludge sample taken from different sources with contrasting solid concentrations. Samples were aerated for three minutes at an air flow-rate of 4 L min−1 and foam produced was rated on an arbitrary scale adapted from [54]. | 1 litre graduated cylinder of 60 mm diameter. Air purged through an Elastox-T© rubber diffuser membrane. | [2] |
| Identical aeration conditions to [6] performed on cell culture broth. Foam generation assessed on scale rating from 0 (no foam formed) to 7 (dense stable foam, stable for more than 5 minutes after aeration ceased). | 250 mL measuring cylinder with sintered disc in base | [17] |
| 50 mL of MLSS liquid sample was placed into a glass cylinder. Gas was passed through a sintered disc and the foam produced was then assessed using an arbitrary rating system. | Specially blown cylinder of diameter 40 mm and length 500 mm. Sintered glass disc had a pore size of 40–90 µm | [2] |
| Test performed on combined surfactant and Gordonia spp. containing activated sludge sample. Instantaneous foam heights were recorded every 10 seconds for 10mins and average foam height determined. | 1 litre graduated cylinder and aerated by compressed at rate of 0.11 m3 h−1 through a sintered silica sand diffuser. | [48] |
2. Materials and Methods
2.1. Samples of Activated Sludge
| Greystones WWTP (30,000 pe) | Swords WWTP (60,000 pe) | Leixlip Industrial WWTP (35,000 pe) | Leixlip Domestic WWTP (45,000 pe) | |
|---|---|---|---|---|
| System | Conventional Plug-flow | Extended Aeration | Conventional Plug-flow | Completely Mixed |
| Primary Treatment | Yes | Yes | No | Yes |
| Aeration type | Fine bubble diffused aeration | Fine bubble diffused aeration | Fine bubble diffused aeration | Mechanical |
| Aerobic reactor | Yes | Yes | Yes | Yes |
| Anoxic reactor | No | Yes | Yes | No |
| Industrial wastes treated | 10% | 5% | 70% | 20% |
2.2. Alka-SeltzerFoam Potential and Stability Test

2.3. Foam Potential Aeration Apparatus

2.4. Statistical Analysis
3. Results and Discussion
3.1. Assessing Optimum Conditions for the Sintered Disc Foam Potential Testing Apparatus
3.1.1. Disc porosity
| WWTP | Porosity | Min (mm) | Mean (mm) | Max (mm) | Standard Deviation (SD) | Coefficient of variation (%) | Repeatability (r) |
|---|---|---|---|---|---|---|---|
| Greystones | 0 | 295 | 317 | 350 | 20.2 | 6.4 | 56.0 |
| 1 | 285 | 306 | 330 | 11.5 | 3.8 | 31.9 | |
| 2 | 290 | 306 | 320 | 7.76 | 2.5 | 21.5 | |
| Leixlip (Domestic) | 0 | 210 | 261 | 330 | 23.5 | 9.0 | 56.0 |
| 1 | 240 | 253 | 275 | 8.2 | 3.2 | 31.1 | |
| 2 | 250 | 261 | 270 | 6.2 | 2.8 | 21.5 | |
| Leixlip (Industrial) | 0 | 220 | 251 | 270 | 15.5 | 6.2 | 65.1 |
| 1 | 230 | 252 | 300 | 18.4 | 7.3 | 22.7 | |
| 2 | 230 | 244 | 270 | 8.4 | 3.5 | 17.2 |
3.1.2. Air flow-rate
| WWTP | Flow rate (L min−1) | Min (mm) | Mean (mm) | Max (mm) | Standard Deviation (SD) | Coefficient of variation (%) | Repeatability (r) |
|---|---|---|---|---|---|---|---|
| Greystones | 0.5 | 290 | 306 | 320 | 7.76 | 2.5 | 23.0 |
| 1.0 | 480 | 574 | 700 | 57.4 | 10.0 | 170.0 | |
| 1.5 | 620 | 720 | 820 | 65.1 | 9.0 | 193.9 | |
| Leixlip (Domestic) | 0.1 | 230 | 250 | 300 | 13.6 | 5.4 | 40.4 |
| 0.3 | 230 | 250 | 270 | 9.03 | 3.6 | 26.7 | |
| 0.5 | 250 | 261 | 270 | 6.20 | 2.4 | 18.3 | |
| Leixlip (Industrial) | 0.1 | 230 | 247 | 265 | 8.90 | 3.6 | 26.4 |
| 0.3 | 250 | 268 | 300 | 13.3 | 5.0 | 39.4 | |
| 0.5 | 230 | 244 | 270 | 8.44 | 3.5 | 25.0 | |
| 1.0 | 270 | 282 | 300 | 8.49 | 3.0 | 25.1 |
3.1.3. Sample volume
| WWTP | Volume (mL) | Min (mm) | Mean (mm) | Max (mm) | Standard Deviation (SD) | Coefficient of variation (%) | Repeatability (r) |
|---|---|---|---|---|---|---|---|
| Greystones | 100 | 220 | 296 | 420 | 55.7 | 20.7 | 164.8 |
| 150 | 295 | 308 | 320 | 7.02 | 2.3 | 20.8 | |
| 200 | 420 | 455 | 525 | 37.4 | 8.2 | 111.0 | |
| Leixlip (Domestic) | 100 | 140 | 199 | 215 | 17.8 | 9.0 | 52.8 |
| 150 | 250 | 260 | 270 | 6.11 | 2.4 | 18.0 | |
| 200 | 285 | 300 | 320 | 8.55 | 2.9 | 25.3 | |
| Leixlip (Industrial) | 100 | 170 | 191 | 210 | 12.1 | 6.3 | 35.9 |
| 150 | 230 | 244 | 270 | 9.30 | 3.8 | 27.6 | |
| 200 | 250 | 272 | 280 | 7.72 | 2.8 | 22.9 |
3.2. Assessing Optimum Conditions for Alka-Seltzer Foaming Method
3.2.1. Use of wire cage
3.2.2. Effect of storage
| Time Period (Hours) | Number of replicates | Foam potential (mm) (AERATED) | Foam Potential (mm) (NON-AERATED) | Significance (α = 0.05) |
|---|---|---|---|---|
| 0 | 10 | 211 ± 12.7 | ||
| (17.8) | ||||
| 4 | 10 | 258 ± 9.7 | 253 ± 14.0 | p > 0.05 |
| (13.8) | (19.6) | |||
| 8 | 10 | 262 ± 7.6 | 294 ± 17.5 | P < 0.05 |
| (10.6) | (24.3) | |||
| 12 | 10 | 310 ± 10.9 | 271 ± 6.0 | P < 0.01 |
| (15.2) | (8.3) | |||
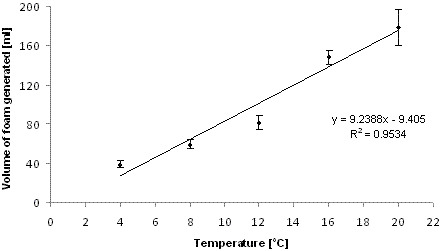
3.2.3. Effect of temperature

3.2.4. Sample volume
3.2.5. Influence of MLSS concentration

| Foam Potential | |||||
|---|---|---|---|---|---|
| MLSS (g L-1) | Number of replicates (n) | Mean Foam potential (mm) (± 95% CI) | Standard Deviation | Coefficient of variation (%) | Repeatability (r) |
| 1.84 | 10 | 293 ± 3.0 | 4.22 | 1.4 | 13.8 |
| 3.57 | 10 | 300 ± 3.6 | 4.97 | 1.7 | 15.9 |
| 4.95 | 10 | 305 ± 2.6 | 3.69 | 1.2 | 11.8 |
3.3. Comparison of Foam Potential between Alka-Seltzer and Sintered Disc Tests
| Leixlip Domestic WWTP | |||||||
| Test Method | Sample Size (n) | Min (%) | Mean (%) ± 95% CI | Max (%) | Standard Deviation (SD) | Coefficient of variation (%) | Repeatability (r) |
| Air test | 20 | 66.7 | 74.0 ± 1.8 | 80.0 | 4.13 | 5.6 | 12.2 |
| Alka-Seltzer | 20 | 44.0 | 73.3 ± 4.1 | 84.0 | 9.27 | 12.6 | 27.4 |
| Leixlip Industrial WWTP | |||||||
| Air test | 20 | 53.3 | 62.3 ± 2.6 | 80.0 | 5.63 | 9.0 | 16.7 |
| Alka-Seltzer | 20 | 32.0 | 49.9 ± 4.2 | 64.0 | 9.63 | 15.0 | 28.5 |
4. Conclusions
- The optimum operating conditions for the sintered disc method are a porosity of 40 to 100 µm (i.e., porosity disc size 2), an air flow-rate of 0.5 L min−1 and a sludge sample volume of 150 mL.
- The application of the wire cage greatly improved the level of precision obtained when performing the Alka-Seltzer test, although the volume of foam produced was reduced by the more controlled release of gas.
- A strong positive linear correlation was found between foam potential results obtained from the Alka-Seltzer test and temperature in the range investigated (4–20 °C). Therefore for comparative purposes the test should be carried out at a prescribed temperature, while for operational use it should be carried out at the same temperature within the aeration basin.
- The Alka-Seltzer method is also affected by mixed liquor solids concentration with non-linear relationships recorded for different sludges. For comparative research then consideration should be given to expressing foam potential at a fixed MLSS concentration (e.g., 3.5 g L−1).
- Based on the mixed liquors used in this work, when measuring foam potential the sintered disc method produces results with better repeatability than the Alka-Seltzer test.
- The Alka-seltzer test also has inherent problems related to the rate of gas released from the tablets as they dissolve which controls the ultimate volume of gas produced.
- The characteristics of the sintered disc test, involving more complex and specialised equipment renders it inappropriate in most cases for use at plant level. Therefore, for routine operational monitoring the Alka-Seltzer test is more appropriate at the operational MLSS and temperature.
| MLSS | mixed liquor suspended solids |
| n | number of replicates |
| r | repeatability of test method |
| RAS | returned activated sludge |
| TSS | total suspended solids |
| Ø | porosity of sintered disc |
| WWTP | waste water treatment plant |
| Zα and Zβ | significance level of Type I and Type II errors |
Acknowledgements
References
- Pipes, W. Actinomycete scum production in activated sludge processes. J. Water Poll. Contr. Fed. 1978, 50, 628–634. [Google Scholar]
- Hug, T. Characterization and Controlling of Foam and Scum in Activated Sludge Systems. PhD Thesis, Swiss Federal Institute of Technology Zurich, Dübendorf, Switzeland, 2006. [Google Scholar]
- Kragelund, C.; Nilsson, B.; Eskilsson, K.; Bøgh, A.; Nielsen, P. Full-scale control of Mycolata foam by FEX-120 addition. Water Sci. Technol. 2010, 61, 2443. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.; de Leon, C.; Radke, C.; Jenkins, D. The Role of Dispersed Nocardioform Filaments in Activated Sludge Foaming. Water Environ. Res. 2010, 82, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ovez, S.; Ors, C.; Murat, S.; Orhon, D. Effect of hypochloride on microbial ecology of bulking and foaming activated sludge treatment for tannery wastewater. J. Environ. Sci. Health A 2006, 41, 2163–2174. [Google Scholar] [CrossRef]
- Heard, J.; Harvey, E.; Johnson, B.; Wells, J.; Angove, M. The effect of filamentous bacteria on foam production and stability. Colloid. Surf. B. Biointerf. 2008, 63, 21–26. [Google Scholar] [CrossRef]
- Davenport, R.; Curtis, T.; Goodfellow, M.; Stainsby, F.; Bingley, M. Quantitative use of fluorescent in situ hybridization to examine relationships between mycolic acid-containing actinomycetes and foaming in activated sludge plants. Appl. Environ. Microbiol. 2000, 66, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kerley, S.; Forster, C. Extracellular polymers in activated sludge and stable foams. J. Chem. Technol. Biotechnol. 1995, 62, 401–404. [Google Scholar] [CrossRef]
- Nam, S.; Chun, J.; Kim, S.; Kim, W.; Zakrzewska-Czerwinska, J.; Goodfellow, M. Tsukamurella spumae sp. nov., a novel actinomycete associated with foaming in activated sludge plants. Syst. Appl. Microbiol. 2003, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.; Mishima, I. Measurement of foam quality of activated sludge in MBR process. Acta Hydroch. Hydrob. 2005, 33, 232–239. [Google Scholar] [CrossRef]
- Lemmer, H.; Lind, G.; Müller, E.; Schade, M.; Ziegelmayer, B. Scum in activated sludge plants: Impact of non-filamentous and filamentous bacteria. Acta Hydroch. Hydrob. 2000, 28, 34–40. [Google Scholar] [CrossRef]
- Guitián, J.; Joseph, D. Foaminess Measurements Using A Shaker Bottle; Department of Aerospace Engineering and Mechanics, University of Minnesota: Minneapolis, MN, July 1996. [Google Scholar]
- Pugh, R. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Soddell, J.; Seviour, R. Microbiology of foaming in activated sludge plants. J. Appl. Microbiol. 1990, 69, 145–176. [Google Scholar]
- Müller, E.; Lind, G.; Lemmer, H.; Wilderer, P. Population structure and chemical EPS analyses of activated sludge and scum. Acta Hydroch. Hydrob. 2005, 33, 189–196. [Google Scholar] [CrossRef]
- Nielsen, P.; Roslev, P.; Dueholm, T.; Nielsen, J. Microthrix parvicella, a specialized lipid consumer in anaerobic-aerobic activated sludge plants. Water Sci. Technol. 2002, 46, 73. [Google Scholar] [PubMed]
- Stratton, H.; Brooks, P.; Griffiths, P.; Seviour, R. Cell surface hydrophobicity and mycolic acid composition of Rhodococcus strains isolated from activated sludge foam. J. Ind. Microbiol. Biotechnol. 2002, 28, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wanner, J. Stable foams and sludge bulking: the largest remaining problems (Abridged). Water Environ. J. 1998, 12, 368–374. [Google Scholar] [CrossRef]
- Blackall, L.; Marshall, K. The mechanism of stabilization of actinomycete foams and the prevention of foaming under laboratory conditions. J. Ind. Microbiol. Biotechnol. 1989, 4, 181–187. [Google Scholar]
- Pugh, R. Experimental techniques for studying the structure of foams and froths. Adv. Colloid Interface Sci. 2005, 114, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Türkman, A. Effect of biosurfactant on 2, 4-dichlorophenol biodegradation in an activated sludge bioreactor. Process Biochem. 2005, 40, 2745–2749. [Google Scholar] [CrossRef]
- Jenkins, D.; Richard, M.; Daigger, G. Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems, 3rd ed.; IWA Publishing: London, UK, 2004; pp. 132–147. [Google Scholar]
- Di Bella, G.; Torregrossa, M.; Viviani, G. The role of EPS concentration in MBR foaming: Analysis of a submerged pilot plant. Bioresour. Technol. 2011, 102, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Pujol, R.; Duchene, P.; Schetrite, S.; Canler, J. Biological foams in activated sludge plants: characterization and situation. Water Res. 1991, 25, 1399–1404. [Google Scholar] [CrossRef]
- Comas, J.; Rodríguez-Roda, I.; Gernaey, K.; Rosén, C.; Jeppsson, U.; Poch, M. Risk assessment modelling of microbiology-related solids separation problems in activated sludge systems. Environ. Model. Software 2008, 23, 1250–1261. [Google Scholar] [CrossRef]
- Wilson, A. Experimental techniques for the characterization of foams. In Foams: Theory, Measurements, and Applications; Prud’homme, R.K., Khan, A.D., Eds.; Marcel Dekker: New York, NY, USA, 1995; p. 243. [Google Scholar]
- Ekserova, D.R.; Kruglëiìakov, P.M. Foam and Foam Films : Theory, Experiment, Application; MÖbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 556–561. [Google Scholar]
- Weaire, D.L.; Hutzler, S. The Physics of Foams; Clarendon Press: Oxford, UK, 1999; pp. 51–52. [Google Scholar]
- Sebba, F. Foams and Biliquid Foams—Aphrons; Wiley: Chichester, UK, 1987; p. 30. [Google Scholar]
- Kelly, W.; Borza, P. Foam test method. J. Am. Oil Chem. Soc. 1966, 43, 364–365. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Malysa, K. Simple and generally applicable method of determination and evaluation of foam properties. J. Surfactants Deterg. 2003, 6, 69–74. [Google Scholar] [CrossRef]
- Pinazo, A.; Pérez, L.; Infante, M.; Franses, E. Relation of foam stability to solution and surface properties of gemini cationic surfactants derived from arginine. Colloids Surf. Physicochem. Eng. Aspects 2001, 189, 225–235. [Google Scholar] [CrossRef]
- Ross, J.; Miles, G. An apparatus for comparison of foaming properties of soaps and detergents. J. Am. Oil Chem. Soc. 1941, 18, 99–102. [Google Scholar]
- Schramm, L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2005; p. 41. [Google Scholar]
- Bikerman, J. The unit of foaminess. Trans. Faraday Soc. 1938, 34, 634–638. [Google Scholar] [CrossRef]
- Baniel, A.; Fains, A.; Popineau, Y. Foaming properties of egg albumen with a bubbling apparatus compared with whipping. J. Food Sci. 1997, 62, 377–381. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Takahashi, A.; Matsudomi, N.; Kobayashi, K. Determination of foa ming properties of proteins by conductivity measurements. J. Food Sci. 1983, 48, 62–65. [Google Scholar] [CrossRef]
- Sezgin, M.; Jenkins, D.; Parker, D. A unified theory of filamentous activated sludge bulking. J. Water Pollut. Contr. Fed. 1978, 50, 362–381. [Google Scholar]
- De los Reyes, F.; Raskin, L. Role of filamentous microorganisms in activated sludge foaming: relationship of mycolata levels to foaming initiation and stability. Water Res. 2002, 36, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Kocianova, E.; Foot, R.; Forster, C. Physicochemical aspects of activated sludge in relation to stable foam formation. Water Environ. J. 1992, 6, 342–350. [Google Scholar] [CrossRef]
- Pretorius, W.; Laubscher, C. Control of biological scum in activated sludge plants by means of selective flotation. Water Sci. Technol. 1987, 19, 1003. [Google Scholar]
- Goddard, A.; Forster, C. Stable foams in activated sludge plants. Enzyme Microb. Technol. 1987, 9, 164–168. [Google Scholar] [CrossRef]
- Khan, A.; Kocianova, E.; Forster, C. Activated sludge characteristics in relation to stable foam formation. J. Chem. Technol. Biotechnol. 1991, 52, 383–392. [Google Scholar] [CrossRef]
- Hladikova, K.; Ruzickova, I.; Klucova, P.; Wanner, J. An investigation into studying of the activated sludge foaming potential by using physicochemical parameters. Water Sci. Technol. 2002, 46, 525. [Google Scholar] [PubMed]
- Torregrossa, M.; Viviani, G.; Vinci, V. Foaming estimation tests in activated sludge systems. Acta Hydroch. Hydrob. 2005, 33, 240–246. [Google Scholar] [CrossRef]
- Ho, C.F.; Jenkins, D. The effect of surfactants on Nocadia foaming in activated sludge. Water Sci. Technol. 1991, 23, 879–887. [Google Scholar]
- Vega-Rodriquez, B.A. Evaluation of Nocardia spp. Presence in Activated Sludge. M.S. Thesis, University of California, Berkley, CA, USA, 1983. [Google Scholar]
- Jolis, D.; Mitch, A.; Marneri, M.; Ho, C. Effects of anaerobic selector hydraulic retention time on biological foam control and en hanced biological phosphorus removal in a pure-oxygen activated sludge system. Water Environ. Res 2007, 79, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cha, D.; Kim, I.; Son, A.; Ahn, K. Fatty Acid Methyl Ester (FAME) Technology for Monitoring Biological Foaming in Acivated Sludge: Full Scale Plant Verification. Environ. Technol. 2008, 29, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Oerther, D.; De Los Reyes, F. Quantifying filamentous microorganisms in activated sludge before, during, and after an incident of foaming by oligonucleotide probe hybridizations and antibody staining. Water Res. 2001, 35, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Lind, G.; Lemmer, H.; Wilderer, P. Dosing aluminum chloride to control Microthrix parvicella. Acta Hydroch. Hydrob. 2005, 33, 247–254. [Google Scholar] [CrossRef]
- Tsang, Y.; Sin, S.; Chua, H. Nocardia foaming control in activated sludge process treating domestic wastewater. Bioresour. Technol. 2008, 99, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Blackall, L.; Harbers, A.; Greenfield, P.; Hayward, A. Activated sludge foams: effects of environmental variables on organism growth and foam formation. Environ. Technol. 1991, 12, 241–248. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA (American Public Health Association): Washington, DC, USA, 1998. [Google Scholar]
- BS1752- Laboratory Sintered (Fritted) Filters—Porosity Grading; British Standard Institution: London, UK, 1983.
- Cohen, B.; Lea, R. Essentials of Statistics for the Social and Behavioral Sciences; Wiley: Hoken, NJ, USA; pp. 130–137.
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Caulcutt, R.; Boddy, R. Statistics for Analytical Chemists; Chapman and Hall: London, UK, 1983; p. 44. [Google Scholar]
- Sokal, R.; Rohlf, F. The Principles and Practice of Statistics in Biological Research, 3rd ed.; Freeman and Co: New York, NY, USA, 1995; pp. 261–264. [Google Scholar]
- von Sperling, M. Basic Principles of Wastewater Treatment, 2nd ed.; IWA Publishing: London, UK, 2007; p. 151. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fryer, M.; O’Flaherty, E.; Gray, N.F. Evaluating the Measurement of Activated Sludge Foam Potential. Water 2011, 3, 424-444. https://doi.org/10.3390/w3010424
Fryer M, O’Flaherty E, Gray NF. Evaluating the Measurement of Activated Sludge Foam Potential. Water. 2011; 3(1):424-444. https://doi.org/10.3390/w3010424
Chicago/Turabian StyleFryer, Martin, Eoghan O’Flaherty, and Nicholas F. Gray. 2011. "Evaluating the Measurement of Activated Sludge Foam Potential" Water 3, no. 1: 424-444. https://doi.org/10.3390/w3010424
APA StyleFryer, M., O’Flaherty, E., & Gray, N. F. (2011). Evaluating the Measurement of Activated Sludge Foam Potential. Water, 3(1), 424-444. https://doi.org/10.3390/w3010424