Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations
Abstract
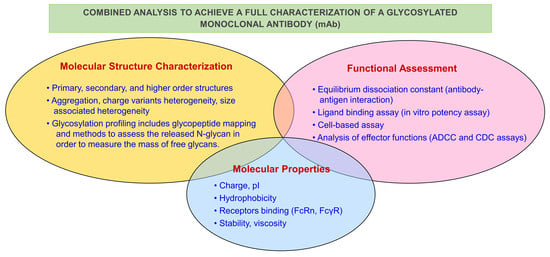
:1. Introduction
2. IgG Glycan Structure and Biosynthesis
2.1. IgG Glycan Structure
2.2. Glycan Biosynthesis in Human Cells
3. N-Glycosylation Impact on mAb Structure and Effector Function
3.1. Impact of Fc Glycosylation on Structure
3.2. Impact of Fc Glycosylation on Immunogenicity
3.3. Impact of Fc Glycosylation on Pharmacokinetics
3.4. Effect of Terminal Mannose on Pharmacokinetics
4. Impact of Fc Glycosylation on Pharmacodynamics
4.1. Sialic Acid
4.2. Terminal Galactose
4.3. Bisecting N-Acetylglucosamine
4.4. Fucose
4.5. High Mannose
5. Glycoengineering
5.1. Cell Glycoengineering
5.2. Chemoenzymatic Glycoengineering
5.3. Glycoengineering for Site-Specific Antibody-Drug Conjugation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fang, J.; Richardson, J.; Du, Z.; Zhang, Z. Effect of Fc-glycan structure on the conformational stability of IgG revealed by hydrogen/deuterium exchange and limited proteolysis. Biochemistry 2016, 55, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Liu, L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 2015, 104, 1866–1884. [Google Scholar] [CrossRef]
- Bakhtiar, R. Therapeutic recombinant monoclonal antibodies. J. Chem. Educ. 2012, 89, 1537–1542. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Z.; Chen, W.; Feng, Y.; Dimitrov, D.S. Crystallizable fragment glycoengineering for therapeutic antibodies development. Front. Immunol. 2017, 8, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T.; et al. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell 2018, 9, 47–62. [Google Scholar] [CrossRef]
- Cymer, F.; Beck, H.; Rohde, A.; Reusch, D. Therapeutic monoclonal antibody N-glycosylation–structure, function and therapeutic potential. Biologicals 2018, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008, 20, 471–478. [Google Scholar] [CrossRef]
- Butters, T.D. Control in the N-linked glycoprotein biosynthesis pathway. Chem. Biol. 2002, 9, 1266–1268. [Google Scholar] [CrossRef] [Green Version]
- Higel, F.; Seidl, A.; Sörgel, F.; Friess, W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Borrok, M.J.; Jung, S.T.; Kang, T.H.; Monzingo, A.F.; Georgiou, G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem. Biol. 2012, 7, 1596–1602. [Google Scholar] [CrossRef] [Green Version]
- Krapp, S.; Mimura, Y.; Jefferis, R.; Huber, R.; Sondermann, P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 2003, 325, 979–989. [Google Scholar] [CrossRef]
- Barbosa, M.D. Immunogenicity of biotherapeutics in the context of developing biosimilars and biobetters. Drug Discov. Today 2011, 16, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Bussat, M.-C.; Lokteff, M.; Klinguer-Hamour, C.; Haeuw, J.-F.; Goetsch, L.; Wurch, T.; Dorsselaer, A.; Corvaia, N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008, 9, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Durocher, Y.; Butler, M. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 2009, 20, 700–707. [Google Scholar] [CrossRef]
- Lam, J.S.; Mansour, M.K.; Specht, C.A.; Levitz, S.M. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J. Immunol. 2005, 175, 7496–7503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biburger, M.; Lux, A.; Nimmerjahn, F. How immunoglobulin G antibodies kill target cells. Adv. Immunol. 2014, 124, 67–94. [Google Scholar] [PubMed]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Liu, T.; Yang, L.; Daus, A.; Crowley, R.; Zhou, Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole–quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal. Biochem. 2007, 364, 8–18. [Google Scholar]
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Stadheim, A.; Hamuro, L.; Pittman, T.; Wang, W.; Zha, D.; Hochman, J.; Prueksaritanont, T. Pharmacokinetics of IgG1 monoclonal antibodies produced in humanized Pichia pastoris with specific glycoforms: A comparative study with CHO produced materials. Biologicals 2011, 39, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.D.; Flynn, G.C. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 2008, 19, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Brown, D.; Reed, C.; Chung, S.; Lutman, J.; Stefanich, E.; Wong, A.; Stephan, J.-P.; Bayer, R. Production, characterization and pharmacokinetic properties of antibodies with N-linked Mannose-5 glycans. MABS 2012, 4, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.; Gebbie, C.; Sweeney, C.; Olszewksi, N.; Smith, J. A phase I, pharmacokinetic (PK) and preliminary efficacy assessment of ALD518, a humanized anti-IL-6 antibody, in patients with advanced cancer (Abstract). J. Clin. Oncol. 2009, 27, 3025. [Google Scholar]
- Abuqayyas, L.; Zhang, X.; Balthasar, J.P. Application of knockout mouse models to investigate the influence of FcγR on the pharmacokinetics and anti-platelet effects of MWReg30, a monoclonal anti-GPIIb antibody. Int. J. Pharm. 2013, 444, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Batra, J.; Rathore, A.S. Glycosylation of monoclonal antibody products: Current status and future. Biotechnol. Prog. 2016, 32, 1091–1102. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamada, T.; Mori, K.; Okazaki, A.; Inoue, M.; Kitajima-Miyama, K.; Kuni-Kamochi, R.; Nakano, R.; Yano, K.; Kakita, S.; et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: The high mannose, hybrid, and complex types. Glycobiology 2006, 17, 104–118. [Google Scholar] [CrossRef]
- Millward, T.A.; Heitzmann, M.; Bill, K.; Längle, U.; Schumacher, P.; Forrer, K. Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biologicals 2008, 36, 41–47. [Google Scholar] [CrossRef]
- Sazinsky, S.L.; Ott, R.G.; Silver, N.W.; Tidor, B.; Ravetch, J.V.; Wittrup, K.D. Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 20167–20172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, T.; Briggs, J.B.; Borge, S.M.; Jones, A.J.S. Species-specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2000, 10, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ambrogelly, A.; Gozo, S.; Katiyar, A.; Dellatore, S.; Kune, Y.; Bhat, R.; Sun, J.; Li, N.; Wang, D.; Nowak, C.; et al. Analytical comparability study of recombinant monoclonal antibody therapeutics. MABS 2018, 10, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.C.; Chen, X.; Liu, Y.D.; Shah, B.; Zhang, Z. Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol. Immunol. 2010, 47, 2074–2082. [Google Scholar] [CrossRef]
- Scallon, B.J.; Tam, S.H.; McCarthy, S.G.; Cai, A.N.; Raju, T.S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 2007, 44, 1524–1534. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Thomann, M.; Schlothauer, T.; Dashivets, T.; Malik, S.; Avenal, C.; Bulau, P.; Rüger, P.; Reusch, D. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS ONE 2015, 10, e0134949. [Google Scholar] [CrossRef]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.-X.; Münz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lünemann, J.D. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef] [Green Version]
- Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory actions of intravenous immunoglobulin. Ann. Rev. Immunol. 2008, 26, 513–533. [Google Scholar] [CrossRef]
- Anthony, R.M.; Ravetch, J.V. A novel role for the IgG Fc glycan: The anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol. 2010, 30, 9–14. [Google Scholar] [CrossRef]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Bondt, A.; Selman, M.H.J.; Deelder, A.M.; Hazes, J.M.; Willemsen, S.P.; Wuhrer, M.; Dolhain, R.J.E.M. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J. Proteome Res. 2013, 12, 4522–4531. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.S.; Jordan, R.E. Galactosylation variations in marketed therapeutic antibodies. MABS 2012, 4, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodoniczky, J.; Zheng, Y.; James, D. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005, 21, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Peschke, B.; Keller, C.W.; Weber, P.; Quast, I.; Lünemann, J.D. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 2017, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Stanley, P. Lectin-resistant CHO glycosylation mutants. Meth. Enzymol. 2006, 416, 159–182. [Google Scholar]
- Davies, J.; Jiang, L.; Pan, L.-Z.; Labarre, M.J.; Anderson, D.; Reff, M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FCγRIII. Biotechnol. Bioeng. 2001, 74, 288–294. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, C.; Brünker, P.; Suter, T.; Moser, S.; Püntener, U.; Umaña, P. Modulation of therapeutic antibody effector functions by glycosylation engineering: Influence of Golgi enzyme localization domain and co-expression of heterologous β1, 4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol. Bioeng. 2006, 93, 851–861. [Google Scholar] [CrossRef]
- Huang, W.; Giddens, J.; Fan, S.-Q.; Toonstra, C.; Wang, L.-X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 2012, 134, 12308–12318. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.L.; Lai, J.; Keck, R.; Oconnell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, R.; Hatanaka, S.; Shoji-Hosaka, E.; Sakurada, M.; Kobayashi, Y.; Uehara, A.; Yokoi, H.; Nakamura, K.; Shitara, K. Enhancement of the antibody-dependent cellular. Clin. Cancer Res. 2004, 10, 6248–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, S.; Misaka, H.; Inoue, M.; Shibata, M.; Nakano, R.; Yamane-Ohnuki, N.; Wakitani, M.; Yano, K.; Shitara, K.; Satoh, M. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to Fc RIIIa. Clin. Cancer Res. 2006, 12, 2879–2887. [Google Scholar] [CrossRef] [Green Version]
- Niwa, R.; Natsume, A.; Uehara, A.; Wakitani, M.; Iida, S.; Uchida, K.; Satoh, M.; Shitara, K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Meth. 2005, 306, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shankara, S.; Roy, A.; Qiu, H.; Estes, S.; Mcvie-Wylie, A.; Culm-Merdek, K.; Park, A.; Pan, C.; Edmunds, T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol. Bioeng. 2007, 99, 652–665. [Google Scholar] [CrossRef]
- Butler, M.; Spearman, M. The choice of mammalian cell host and possibilities for glycosylation engineering. Curr. Opin. Biotechnol. 2014, 30, 107–112. [Google Scholar] [CrossRef]
- Suzuki, E.; Niwa, R.; Saji, S.; Muta, M.; Hirose, M.; Lida, S.; Shiotsu, Y.; Satoh, M.; Shitara, K.; Kondo, M.; et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin. Cancer Res. 2007, 13, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Yamane-Ohnuki, N.; Satoh, M. Production of therapeutic antibodies with controlled fucosylation. MABS 2009, 1, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Urbain, R.; Teillaud, J.L.; Prost, J.F. EMABling antibodies: From feto-maternal allo-immunisation prophylaxis to chronic lymphocytic leukaemia therapy. Med. Sci. 2009, 25, 1141–1144. [Google Scholar]
- Dicker, M.; Strasser, R. Using glyco-engineering to produce therapeutic proteins. Expert Opin. Biol. Ther. 2015, 15, 1501–1516. [Google Scholar] [CrossRef]
- Crowell, C.K.; Grampp, G.E.; Rogers, G.N.; Miller, J.; Scheinman, R.I. Amino acid and manganese supplementation modulates the glycosylation state of erythropoietin in a CHO culture system. Biotechnol. Bioeng. 2006, 96, 538–549. [Google Scholar] [CrossRef]
- Okeley, N.M.; Alley, S.C.; Anderson, M.E.; Boursalian, T.E.; Burke, P.J.; Emmerton, K.M.; Jeffrey, S.C.; Klussman, K.; Law, C.-L.; Sussman, D.; et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 5404–5409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, L.D. Inhibition of N-Linked Glycosylation. Curr. Protoc. Mol. Biol. 1995, 32, 17101–17109. [Google Scholar] [CrossRef]
- Sullivan, F.X.; Kumar, R.; Kriz, R.; Stahl, M.; Xu, G.-Y.; Rouse, J.; Chang, X.-J.; Boodhoo, A.; Potvin, B.; Cumming, D.A. Molecular cloning of human GDP-mannose 4,6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J. Biol. Chem. 1998, 273, 8193–8202. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.F.; Shahreel, W.; Wan, C.; Teo, G.; Hayati, N.; Tay, S.J.; Tong, W.H.; Yang, Y.; Rudd, P.M.; Zhang, P.; et al. Inactivation of GDP-fucose transporter gene (Slc35c1) in CHO cells by ZFNs, TALENs and CRISPR-Cas9 for production of fucose-free antibodies. Biotechnol. J. 2015, 11, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Imai-Nishiya, H.; Mori, K.; Inoue, M.; Wakitani, M.; Lida, S.; Shitara, K.; Satoh, M. Double knockdown of alpha 1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: A new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giddens, J.P.; Wang, L.-X. Chemoenzymatic Glyco-engineering of monoclonal antibodies. Methods Mol. Biol. 2015, 1321, 375–387. [Google Scholar] [PubMed] [Green Version]
- Kurogochi, M.; Mori, M.; Osumi, K.; Tojino, M.; Sugawara, S.-I.; Takashima, S.; Hirose, Y.; Tsukimura, W.; Mizuno, M.; Amano, J.; et al. Glycoengineered monoclonal antibodies with homogeneous glycan (M3, G0, G2, and A2) using a chemoenzymatic approach have different affinities for FcγRIIIa and variable antibody-dependent cellular cytotoxicity activities. PLoS ONE 2015, 10, e0132848. [Google Scholar] [CrossRef] [Green Version]
- Umekawa, M.; Huang, W.; Li, B.; Fujita, K.; Ashida, H.; Wang, L.-X.; Yamamoto, K. Mutants of mucor hiemalis endo-β-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J. Biol. Chem. 2007, 283, 4469–4479. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Tong, X.; Yang, Q.; Giddens, J.P.; Wang, L.-X. Glycosynthase mutants of endoglycosidase S2 show potent transglycosylation activity and remarkably relaxed substrate specificity for antibody glycosylation remodeling. J. Biol. Chem. 2016, 291, 16508–16518. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-X.; Tong, X.; Li, C.; Giddens, J.P.; Li, T. Glycoengineering of Antibodies for Modulating Functions. Annu. Rev. Biochem. 2019, 88, 433–459. [Google Scholar] [CrossRef]
- Bumbaca, D.; Boswell, C.A.; Fielder, P.J.; Khawli, L.A. Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J. 2012, 14, 554–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferis, R. Glycosylation as a strategy to improve antibody based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Manoli, H.; Jaw, S.; Frutoz, K.; Epstein, A.L.; Theil, F.P.; Khawli, L.A. Unraveling the effect of immunogenicity response on the PK/PD, efficacy, and safety of biologics. J. Immunol. Res. 2016, 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Khawli, L.A.; Purushothama, S.; Theil, F.P.; Partridge, M. Recent advances in assessing immunogenicity of therapeutic proteins: Impact on biotherapeutic development. J. Immunol. Res. 2016, 2016, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Liu, H. Macro-and micro-heterogeneity of natural and recombinant IgG antibodies. Antibodies 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duivelshof, B.L.; Jiskoot, W.; Beck, A.; Veuthey, J.-L.; Guillarme, D.; D’Atri, V. Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications. Anal. Chim. Acta 2019, 1089, 1–18. [Google Scholar] [CrossRef] [Green Version]









| Fc-Glycans | Potential Effects | References |
|---|---|---|
| Fucose |
Absence of core fucose enhances:
| [48,51,52,53,54] |
| Galactose | Enhances antibody binding to C1q and CDC | [44,45] |
| Sialic acid |
| [36,39] [35,36] [18,19,20] [3,25] |
| High Mannose |
| [17,25,29] [55] [29,55] |
| Bisecting GlcNAc | Increases FcγRIIIa binding and ADCC activity | [44,47,48,49] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. https://doi.org/10.3390/antib9020022
Boune S, Hu P, Epstein AL, Khawli LA. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies. 2020; 9(2):22. https://doi.org/10.3390/antib9020022
Chicago/Turabian StyleBoune, Souad, Peisheng Hu, Alan L. Epstein, and Leslie A. Khawli. 2020. "Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations" Antibodies 9, no. 2: 22. https://doi.org/10.3390/antib9020022
APA StyleBoune, S., Hu, P., Epstein, A. L., & Khawli, L. A. (2020). Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies, 9(2), 22. https://doi.org/10.3390/antib9020022