Low-Temperature Crystal Chemistry of Hingganite-(Y), from the Wanni Glacier, Switzerland
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Composition
3.2. Crystal Structure Refinement
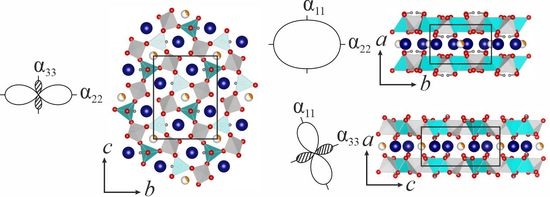
3.3. Thermal Behavior of Hingganite-(Y)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machatschki, F. Zur Frage der Struktur und Konstitution der Feldspate. Zentralbl. Mineral. 1928, A, 97–100. [Google Scholar]
- Bragg, W.L. The structure of silicates. Z. Kristallogr. 1930, 74, 237–305. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Huminicki, D.M.C. The crystal chemistry of beryllium. In Beryllium: Mineralogy, Petrology and Geochemistry; Grew, E.S., Ed.; Reviews in Mineralogy & Geochemistry; de Gruyter GmbH: Berlin, Germany, 2002; Volume 50, pp. 333–403. [Google Scholar]
- Bačík, P.; Miyawaki, R.; Atencio, D.; Camara, F.; Fridrichová, J. Nomenclature of gadolinite supergroup. Eur. J. Mineral. 2017, 29, 1067–1082. [Google Scholar] [CrossRef] [Green Version]
- Škoda, R.; Plasil, J.; Copjakova, R.; Novak, M.; Jonsson, E.; Vasinova Galiova, M.; Holtstam, D. Gadolinite-(Nd), a new member of the gadolinite supergroup from the Fe-REE deposits of Bastnas-type, Sweden. Min. Mag. 2018, 82, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Pršek, J.; Ondrejka, M.; Bačík, P.; Budzyń, B.; Uher, P. Metamorphic-hydrothermal REE minerals in the Bacuch magnetite deposit, Western Carpathians, Slovalia: (Sr,S)-rich monacite-(Ce) and Nd-dominant hingganite. Can. Mineral. 2010, 48, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Kasatkin, A.V.; Nestola, F.; Skoda, R.; Chukanov, N.V.; Agakhanov, A.A.; Belakovsky, D.I.; Lanza, A.; Hola, M. Hingganite-(Nd), IMA 2019-028. CNMNC Newsletter Min. Mag. 2019, 50, 619. [Google Scholar]
- Voloshin, A.V.; Pakhomovskii, Y.A.; Men’shikov, Y.P.; Povarennykh, A.S.; Matvienko, E.N.; Yakubovich, O.V. Ytterbium hingganite—A new mineral from amazonite pegmatites of the Kola Peninsula. Dokl Akad Nauk SSSR 1983, 270, 1188–1192. (In Russian) [Google Scholar]
- Yakubovich, O.V.; Matvienko, E.N.; Voloshin, A.V.; Simonov, M.A. The crystal structure of hingganite-(Yb) (Y0.51Ln0.36Ca0.13)Fe0.065Be(SiO4)(OH). Sov. Phys. Crystallogr. 1983, 28, 269–271. [Google Scholar]
- Raade, G.; Kolitsch, U.; Husdal, T.A. Si-rich bergslagite from a granitic pegmatite at Tennvatn, north Norway. GFF 2006, 128, 65–68. [Google Scholar] [CrossRef]
- Miyawaki, R.; Matsubara, S.; Yokoyama, K.; Okamoto, A. Hingganite-(Ce) and hingganite-(Y) from Tahara, Hirukawa-mura, Gifu Prefecture, Japan: The description on a new mineral species of the Ce-analogue of hingannite-(Y) with a refinement of the crystal structure of hingganite-(Y). J. Miner. Petrol. Sci. 2007, 102, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lyalina, L.M.; Selivanova, E.A.; Savchenko, Y.E.; Zozulya, D.R.; Kadyrova, G.I. Minerals of the gadolinite-(Y)-hingganite-(Y) series in the alkali granite pegmatites of the Kola Peninsula. Geol. Ore Depos. 2014, 56, 675–684. [Google Scholar] [CrossRef]
- Pezzotta, F.; Diella, V.; Guastoni, A. Chemical and paragenetic data on gadolinite-group minerals from Baveno and Cuasso al Monte, southern Alps, Italy. Am. Mineral. 1999, 84, 782–789. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Hingganite-(Y) from a small aplite vein in granodiorite from Oppach, Lusatian Mts., E-Fermany. Miner. Petrol. 2017, 111, 821–826. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Mitchell, R.H.; Ren, M.; Sen, A.K.; Pruseth, K.L. Rinkite, cerianite-(Ce), and hingganite-(Ce) in syenite gneisses from the Sushina Hill Complex, India: Occurrence, compositional data and petrogenetic significance. Min. Mag. 2013, 77, 3137–3153. [Google Scholar] [CrossRef]
- Holtstam, D.; Anderson, U.B. The REE minerals pf the Bastnas-type deposits, south-central Sweden. Can. Mineral. 2007, 45, 1523–1527. [Google Scholar] [CrossRef]
- Macdonald, R.; Baginski, B.; Kartashov, P.; Zozulia, D.; Dzierzanowski, P. Interaction of rare-metal minerals with hydrothermal fluids: Evidence from quartz-epidote metasomatites of the Haldzan Buragtag massif, Mongolian Altai. Can. Mineral. 2015, 53, 1015–1034. [Google Scholar] [CrossRef]
- Majka, J.; Pršek, J.; Budzyń, B.; Bačík, P.; Barker, A.K.; Łodzinski, M. Fluorapatite-hingganite-(Y) coronas as products of fluid induced xenotime-(Y) breakdown in the Skoddefjellet pegmatite, Svalbard. Mineral. Mag. 2011, 75, 159–167. [Google Scholar] [CrossRef]
- Demartin, F.; Pilati, T.; Diella, V.; Gentile, P.; Gramaccioli, C.M. A crystal-chemical investigation of Alpine gadolinite. Can. Mineral. 1993, 31, 127–136. [Google Scholar]
- Rinaldi, R.; Gatta, G.D.; Angel, R.J. Crystal chemistry and low-temperature behavior of datolite: A single crystal X-ray diffraction study. Am. Mineral. 2010, 95, 1413–1421. [Google Scholar] [CrossRef]
- Tarney, J.; Nicol, A.W.; Marriner, G.F. The thermal transformation of datolite, CaBSiO4(OH), to boron-melilite. Min. Mag. 1973, 39, 158–175. [Google Scholar] [CrossRef] [Green Version]
- Kimata, M. Boron behavior in the thermal decomposition of datolite. Neues Jahrb. Mineral. Monatsh. 1978, 58–70. [Google Scholar]
- Perchiazzi, N.; Gualtieri, A.F.; Merlino, S.; Kampf, A.R. The atomic structure of bakerite and its relationship to datolite. Am. Mineral. 2004, 89, 767–776. [Google Scholar] [CrossRef]
- Krzhizhanovskaya, M.G.; Gorelova, L.A.; Bubnova, R.S.; Pekov, I.V.; Krivovichev, S.V. High-temperature crystal chemistry of layered calcium borosilicates: CaBSiO4(OH) (datolite), Ca4B5Si3O15(OH)5 (‘bakerite’) and Ca2B2SiO7 (synthetic analogue of okayamalite). Phys. Chem. Miner. 2018, 45, 463–473. [Google Scholar] [CrossRef]
- Goryainov, S.V.; Krylov, A.S.; Vtyurin, A.N.; Pan, Y. Raman study of datolite CaBSiO4(OH) at simultaneously high pressure and high temperature. J. Raman Spectr. 2015, 46, 177–181. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Pakhomova, A.S.; Aprilis, G.; Dubrovinsky, L.; Krivovichev, S. Pentacoordinated silicon in the high-pressure modification of datolite, CaBSiO4(OH). Inorg. Chem. Front. 2018, 5, 1653–1660. [Google Scholar] [CrossRef]
- Gibson, S.J.; Ehlmann, A.J. Annealing characteristics of metamict gadolinite from Rode Ranch Texas. Am. Mineral. 1970, 55, 288–291. [Google Scholar]
- Janeczek, J.; Eby, R.K. Annealing of radiation damage in allanite and gadolinite. Phys. Chem. Miner. 1993, 19, 343–356. [Google Scholar] [CrossRef]
- Malczewski, D. 57Fe Moessbauer Spectroscopy of the Thermally Recrystallized Fully Metamict Gadolinites; University of Silesia in Katowice: Katowice, Poland, 1999; pp. 68–122. [Google Scholar]
- Malczewski, D.; Janeczek, J.; Popiel, E. Moessbauer studies of fully metamict gadolinite annealed in air. Mol. Phys. Rep. 1998, 22, 111–116. [Google Scholar]
- Malczewski, D.; Janeczek, J. Activation energy of annealed metamict gadolinite from 57Fe Moessbauer spectroscopy. Phys, Chem. Miner. 2002, 29, 226–232. [Google Scholar] [CrossRef]
- Malczewski, D. Structural analysis of radiation in Precambrian gadolinite from Ytterby by 57Fe Moessbauer spectroscopy, transmission electron microscopy and X-ray diffraction. Mat. Sci. Eng. A 2004, 375–377, 1259–1262. [Google Scholar] [CrossRef]
- Demartin, F.; Minaglia, A.; Gramaccioli, C.M. Characterization of gadolinite-group minerals using crystallographic data only: The case of hingganite-(Y) from Cuasso al Monte, Italy. Can. Mineral. 2001, 39, 1105–1114. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Pakhomova, A.S.; Krivovichev, S.V.; Kasatkin, A.V.; Dubrovinsky, L.S. Compressibility of hingganite-(Y): High-pressure single crystal X-ray diffraction study. Phys. Chem. Miner. 2020, in press. [Google Scholar] [CrossRef]
- Graeser, S. Asbecasit und Cafarsit, zwei neue Mineralien aus dem Binnatal (Kt. Wallis). Schweiz. Mineral. Petrogr. Mitt. 1966, 46, 367–375. [Google Scholar]
- Graeser, S.; Schwander, H. Gasparite-(Ce) and monazite-(Nd): Two new minerals to the monazite group from the Alps. Schweiz. Mineral. Petrogr. Mitt. 1987, 67, 101–113. [Google Scholar]
- Armbruster, T.; Buhler, C.; Graeser, S.; Stalder, H.A.; Amthauer, G. Cervandonite-(Ce), (Ce,Nd,La)(Fe3+,Fe2+,Ti4+,Al)3SiAs(Si,As)O13, a new Alpine fissure mineral. Schweiz. Mineral. Petrogr. Mitt. 1988, 68, 125–132. [Google Scholar]
- Demartin, F.; Gramaccioli, C.M.; Pilati, T. Paraniite-(Y), a new tungstate arsenate mineral from Alpine fissures. Schweiz. Mineral. Petrogr. Mitt. 1994, 74, 155–160. [Google Scholar]
- Graeser, S.; Schwander, H.; Demartin, F.; Gramaccioli, C.M.; Pilati, T.; Reusser, E. Fetiasite (Fe2+, Fe3+,Ti)3O2[As2O5], a new arsenite mineral: Its description and structure determination. Am. Mineral. 1994, 79, 996–1002. [Google Scholar]
- Guastoni, A.; Pezzota, F.; Vignola, P. Characterization and genetic inferences of arsenates, sulfates and vanadates of Fe, Cu, Pb, Zn from Mount Cervadone (Western Alps, Italy). Period Mineral. 2006, 75, 141–150. [Google Scholar]
- Guastoni, A.; Nestola, F.; Gentile, P.; Zorzi, F.; Alvaro, M.; Lanza, A.; Peruzzo, L.; Schiazza, M.; Casati, N.M. Deveroite-(Ce): A new REE-oxalate from Mount Cervandone, Devero Valley, Western-Central Alps, Italy. Min. Mag. 2013, 77, 3019–3026. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Britvin, S.N.; Perova, E.N.; Brusnitsyn, A.I.; Polekhovsky, Y.S.; Shilovskikh, V.V.; Bocharov, V.N.; van der Burgt, A.; Cuchet, S.; Meisser, N. Gasparite-(La), La(AsO4), a new mineral from Mn ores of the Ushkatyn-III deposit, Central Kazakhstan, and metamorphic rocks of the Wanni glacier, Switzerland. Am. Mineral. 2019, 104, 1469–1480. [Google Scholar] [CrossRef]
- Streckeisen, A.; Wenk, E.; Frey, M. On steep isogradic surfaces in the Simplon area. Contrib. Mineral. Petrol. 1974, 47, 8l–95. [Google Scholar] [CrossRef]
- Steck, A. Le massif du Simplon. Reflexions sur la cinematique des nappes de gneiss. Schweiz. Mineral. Petrogr. Mitt. 1987, 67, 27–45. [Google Scholar]
- Knill, M.D. Geochemistry and Genesis of the Metadolomite-Hosted Pb-Zn-As-Tl-Ba-Deposit and Lengenbach, Binn Valley, Switzerland. Ph.D. Thesis, Swiss Federal Institute of Technology (ETH) Zurich, Zurich, Switzerland, 1995. [Google Scholar]
- Klemm, L.; Pettke, T.; Graeser, S.; Mullis, J.; Kouzmanov, K. Fluid mixing as the cause of sulphide precipitation at Albrunpass, Binn Valley, Central Alps. Schweiz. Mineral. Petrogr. Mitt. 2004, 84, 189–212. [Google Scholar]
- Hettmann, K.; Kreissig, K.; Rehkämper, M.; Wenzel, T.; Mertz-Kraus, R.; Markl, G. Thallium geochemistry in the metamorphic Lengenbach sulfide deposit, Switzerland: Thallium-isotope fractionation in a sulfide melt. Am. Mineral. 2014, 99, 793–803. [Google Scholar] [CrossRef]
- Bergomi, M.A.; Dal Piaz, G.V.; Malusà, M.G.; Monopoli, B.; Tunesi, A. The Grand St Bernard-Briançonnais nappe system and the Paleozoic inheritance of the Western Alps unraveled by zircon U-Pb dating. Tectonics 2017, 36, 2950–2972. [Google Scholar] [CrossRef] [Green Version]
- Stalder, H.A.; Wagner, A.; Graeser, S.; Stuker, P. Mineralienlexicon der Schweiz; Verlag Wepf & Co. AG: Basel, Switzerland, 1998; 608p. [Google Scholar]
- Cuchet, S.; Crumbach, M.; van der Burgt, A.; Vanini, F.; Loranth, C.; Mestrom, P.; Antonysen, E.; Meisser, N.; Roth, P. Mineralogische Topografie der Schweiz und angrenzender Regionen. 1. Teil—Binntal (CH)—Veglia (I)—Devero (I). Schweiz. Strahler. 2019, 1, 2–17. [Google Scholar]
- Agilent. CrysAlis PRO; Agilent Technologies: Yarnton, UK, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112. [Google Scholar] [CrossRef] [Green Version]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (Theta to Tensor-TTT). Glass Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Quantitative analysis of homogeneous or stratified microvolumes applying the model BPAP. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Springer Verlag: New York, NY, USA, 1991. [Google Scholar]
- Nasdala, L.; Beyssac, O.; Schopf, J.W.; Bleisteiner, B. Application of Raman-based images in the Earth sciences. In Raman Imaging—Techniques and Applications; Zoubrir, A., Ed.; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–187. [Google Scholar]
- Lenz, C.; Nasdala, L.; Talla, D.; Hauzenberger, C.; Seitz, R.; Kolitsch, U. Laser-induced REE3+ photoluminescence of selected accessory minerals –An “advantageous artefact” in Raman spectroscopy. Chem. Geol. 2015, 415, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K.; Kristiansen, R.; Pekov, I.V.; Belakovskiy, D.I.; Van, K.V.; Bychkova, Y.V.; Britvin, S.N. Crystal structure of the OH-dominant gadolinite-(Y) analogue (Y,Ca)2(Fe,□)Be2Si2O8(OH,O)2 from Heftetjern pegmatite. Acta Crystallogr. 2017, B73, 899–906. [Google Scholar]
- Frost, R.L.; Xi, Y.; Scholz, R.; Lima, R.M.F.; Horta, L.F.C.; Lopez, A. Thermal analysis and vibrational spectroscopic characterization of the boro silicate mineral datolite–CaBSiO4(OH). Spectrochim. Acta A 2013, 115, 376–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, R.L.; Scholz, R.; López, A.; Xi, Y.; de Siqueira Queiroz, C.; Belotti, F.M.; Cândido Filho, M. Raman, infrared and near-infrared spectroscopic characterization of the herderite–hydroxylherderite mineral series. Spectrochim. Acta A 2014, 118, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeister, A.M.; Hoering, T.C.; Virgo, D. Vibrational spectroscopy of beryllium aluminosilicates: Heat capacity calculations from band assignments. Phys. Chem. Mineral. 1987, 14, 205–224. [Google Scholar] [CrossRef]
- Bačík, P.; Fridrichová, J.; Uher, P.; Pršek, J.; Ondrejka, M. The crystal chemistry of gadolinite-datolite group silicates. Can. Mineral. 2014, 52, 625–642. [Google Scholar] [CrossRef]
- Camara, F.; Oberti, R.; Ottolini, L.; Della Ventura, G.; Bellatreccia, F. The crystal chemistry of Li in gadolinite. Am. Mineral. 2008, 93, 996–1004. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Filatov, S.K. Strong anisotropic thermal expansion in borates. Phys. Stat. Sol. 2008, 245, 2469–2476. [Google Scholar] [CrossRef]
- Morosin, B. Structure and thermal expansion of beryl. Acta Cryst. 1972, B28, 1899–1903. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. The structure of danalite at high temperature obtained from synchrotron radiation and Rietveld refinements. Can. Mineral. 2003, 41, 1413–1422. [Google Scholar] [CrossRef]
- Tang, J.-J.; Liu, X.; Xiong, Z.-H.; He, Q.; Sean, R.S.; Wang, H.-J. High temperature X-ray diffraction, DSC-TGA, polarized FTIR and high pressure Raman spectroscopy studies on euclase. Bull. Mineral. Petrol. Geochem. 2014, 33, 289–298. [Google Scholar]
- Hazen, R.M.; Finger, L.W. High-temperature crystal chemistry of phenakite (Be2SiO4) and chrysoberyl (BeAl2O4). Phys. Chem. Miner. 1987, 14, 426–434. [Google Scholar] [CrossRef]
- Henderson, C.M.B.; Taylor, D. Thermal expansion behavior of beryllonite [Na(BePO4)] and trimerite [CaMn(BeSiO4)3]. Min. Mag. 1984, 48, 431–436. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. Tugtupite: High-temperature structures obtained from in situ synchrotron diffraction and Rietveld refinements. Am. Mineral. 2004, 89, 492–497. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Filatov, S.K. Vysokotemperaturnaya Kristallokhimiya Boratov i Borosilikatov (High Temperature Crystal Chemistry of Borates and Borosilicates); Nauka: St. Petersburg, Russia, 2008. (In Russian) [Google Scholar]
- Uher, P.; Ondrejka, M.; Konečný, P. Magmatic and post-magmatic Y-REE-Th phosphate, silicate and Nb-Ta-Y-REE oxide minerals in A-type metagranite: An example from the Turcok massif, the Western Carpathians, Slovakia. Mineral. Mag. 2009, 73, 1009–1025. [Google Scholar] [CrossRef]
- Jambor, J.L.; Roberts, A.C.; Griece, J.D.; Birkett, T.C.; Groat, L.A.; Zajac, S. Gerenite-(Y), (Ca,Na)2(Y,REE)3Si6O18·2H2O, a new mineral species, and an associated Y-bearing gadolinite-group mineral, from the Strange Lake peralkaline complex, Quebec-Labrador. Can. Mineral. 1998, 36, 793–800. [Google Scholar]
- Pekov, I.V.; Voloshin, A.V.; Pushcharovskii, D.Y.; Rastsvetaeva, R.K.; Chukanov, N.V.; Belokovskii, D.I. New data on calcybeborosilite-(Y) (REE,Ca)2(B,Be)2(SiO4)2(OH,O)2. Vestnik Moskovskogo Universiteta Geologiya 2000, 55, 62–70. [Google Scholar]
- Grew, E.S. Borosilicates (exclusive of tourmaline) and boron in rock-forming minerals in metamorphic enviroments. In Boron: Mineralogy, Petrology and Geochemistry; Grew, E.S., Anowitz, L.M., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 1996; Volume 33, pp. 387–502. [Google Scholar]
- Hazen, R.M.; Finger, L.W. Comparative Crystal Chemistry: Temperature, Pressure, Composition and the Variation of Crystal Structure; John Wiley and Sons Ltd.: London, UK, 1982; p. 231. [Google Scholar]
- Filatov, S.K. Vysokotemperaturnaya Kristallokhimiya (High Temperature Crystal Chemistry); Nedra: Leningrad, Russia, 1990. (In Russian) [Google Scholar]









| Constituent | Mean, wt.% | Range, wt.% | Site | Constituent | *A = 2 | *T = 2 |
|---|---|---|---|---|---|---|
| Mean, apfu | Mean, apfu | |||||
| CaO | 8.87(5) | 8.20–9.35 | A | Ca | 0.68 | 0.68 |
| Y2O3 | 26.70(30) | 26.48–27.12 | Y | 1.02 | 1.02 | |
| La2O3 | 0.75(4) | 0.69–0.78 | La | 0.02 | 0.02 | |
| Ce2O3 | 0.64(8) | 0.31–0.90 | Ce | 0.02 | 0.02 | |
| Pr2O3 | 0.15(3) | 0.03–0.23 | Pr | 0.00 | 0.00 | |
| Nd2O3 | 1.15(3) | 0.78–1.52 | Nd | 0.03 | 0.03 | |
| Sm2O3 | 0.87(4) | 0.64–1.15 | Sm | 0.02 | 0.02 | |
| Eu2O3 | 0.07(2) | 0.06–0.09 | Eu | 0.00 | 0.00 | |
| Gd2O3 | 1.74(5) | 1.25–2.27 | Gd | 0.04 | 0.04 | |
| Tb2O3 | 0.82(4) | 0.67–0.96 | Tb | 0.02 | 0.02 | |
| Dy2O3 | 2.15(2) | 1.98–2.31 | Dy | 0.05 | 0.05 | |
| Ho2O3 | 0.61(4) | 0.42–0.69 | Ho | 0.01 | 0.01 | |
| Er2O3 | 1.15(3) | 1.00–1.34 | Er | 0.03 | 0.03 | |
| Tm2O3 | 0.20(3) | 0.05–0.28 | Tm | 0.00 | 0.00 | |
| Yb2O3 | 0.56(3) | 0.47–0.73 | Yb | 0.01 | 0.01 | |
| ThO2 | 2.79(7) | 2.16–3.45 | Th | 0.05 | 0.05 | |
| FeO | 6.55(9) | 6.42–6.79 | M | Fe | 0.39 | 0.39 |
| MgO | 0.03(1) | 0.01–0.05 | Mg | 0.00 | 0.00 | |
| SiO2 | 27.83(10) | 27.32–28.27 | T + Q | Si | 1.99 | 2.00 |
| As2O5 | 0.13(5) | 0.08–0.16 | As | 0.00 | 0.00 | |
| BeOcalc ** | 6.64 | Be | 1.14 | 1.15 | ||
| B2O3calc ** | 6.97 | B | 0.86 | 0.86 | ||
| H2Ocalc *** | 1.98 | ϕ | OH **** | 0.95 | 1.03 | |
| O **** | 1.05 | 0.97 | ||||
| ACa/sum of A-site cations | 0.34 | 0.34 | ||||
| TSi/sum of T-site cations | 1.00 | 1.00 | ||||
| Total | 99.36 | sum of M-site cations | 0.39 | 0.39 | ||
| Temperature, °C | −173 | −143 | −113 | −83 | −53 | −23 | +7 |
| Temperature point | T01 | T02 | T03 | T04 | T05 | T06 | T07 |
| Space group, Z | P21/c, 2 | ||||||
| a, Å | 4.7469(2) | 4.7458(2) | 4.7482(3) | 4.7491(3) | 4.7498(3) | 4.7493(3) | 4.7494(3) |
| b, Å | 7.5919(3) | 7.5932(3) | 7.5917(3) | 7.5927(3) | 7.5956(3) | 7.5995(3) | 7.6003(3) |
| c, Å | 9.8841(3) | 9.8844(3) | 9.8801(4) | 9.8789(4) | 9.8822(4) | 9.8822(3) | 9.8794(4) |
| β, ˚ | 90.249(3) | 90.246(3) | 90.293(4) | 90.297(4) | 90.290(4) | 90.294(4) | 90.304(4) |
| Volume, Å3 | 356.20(2) | 356.19(2) | 356.15(3) | 356.25(3) | 356.52(3) | 356.67(3) | 356.61(3) |
| Data collection | |||||||
| Wavelength, Å | 0.71073 | ||||||
| Max. θ° | 32.036 | 32.033 | 32.433 | 32.090 | 32.418 | 32.407 | 32.407 |
| Index ranges | −4 ≤ h ≤ 7 −11 ≤ k ≤ 9 −13 ≤ l ≤ 14 | −7 ≤ h ≤ 4 −11 ≤ k ≤ 9 −14 ≤ l ≤ 13 | −7 ≤ h ≤ 5 −11 ≤ k ≤ 4 −13 ≤ l ≤ 14 | −7 ≤ h ≤ 5 −11 ≤ k ≤ 4 −13 ≤ l ≤ 14 | −7 ≤ h ≤ 5 −11 ≤ k ≤ 4 −13 ≤ l ≤ 14 | −7 ≤ h ≤ 5 −11 ≤ k ≤ 4 −13 ≤ l ≤ 14 | −7 ≤ h ≤ 5 −11 ≤ k ≤ 4 −13 ≤ l ≤ 14 |
| No. meas. refl. | 1960 | 1919 | 2034 | 1985 | 2022 | 2022 | 2018 |
| No. uniq. refl. | 1125 | 1125 | 1116 | 1107 | 1116 | 1117 | 1113 |
| No. obs. refl (I > 2σ(I)) | 1020 | 1029 | 1015 | 1008 | 1014 | 998 | 985 |
| Refinement of the structure | |||||||
| No. of variables | 81 | 79 | 79 | 79 | 79 | 79 | 79 |
| Rint | 0.0372 | 0.0379 | 0.0362 | 0.0382 | 0.0382 | 0.0379 | 0.0360 |
| R1, all data | 0.0537 | 0.0537 | 0.0543 | 0.0540 | 0.0559 | 0.0554 | 0.0556 |
| R1, I > 2σ(I) | 0.0471 | 0.0485 | 0.0485 | 0.0485 | 0.0493 | 0.0481 | 0.0478 |
| wR2, all data | 0.0965 | 0.1000 | 0.1085 | 0.1059 | 0.1083 | 0.1049 | 0.1080 |
| wR2, I > 2σ(I) | 0.0942 | 0.0978 | 0.1064 | 0.1033 | 0.1058 | 0.1023 | 0.1046 |
| GooF | 1.367 | 1.349 | 1.343 | 1.298 | 1.328 | 1.357 | 1.258 |
| Temperature, °C | −173 | −143 | −113 | −83 | −53 | −23 | +7 |
| Temperature point | T01 | T02 | T03 | T04 | T05 | T06 | T07 |
| SiO4 tetrahedra | |||||||
| Si–O3 | 1.592(6) | 1.593(6) | 1.596(6) | 1.594(5) | 1.589(6) | 1.590(6) | 1.591(5) |
| Si–O4 | 1.647(6) | 1.649(6) | 1.644(6) | 1.648(5) | 1.646(6) | 1.644(5) | 1.649(5) |
| Si–O1 | 1.637(6) | 1.637(6) | 1.638(6) | 1.639(6) | 1.639(5) | 1.635(6) | 1.634(5) |
| Si–O5 | 1.644(6) | 1.639(6) | 1.635(6) | 1.630(6) | 1.634(6) | 1.636(6) | 1.629(6) |
| <Si–O> | 1.630 | 1.629 | 1.628 | 1.628 | 1.627 | 1.626 | 1.626 |
| Volume | 2.210 | 2.208 | 2.120 | 2.201 | 2.197 | 2.194 | 2.195 |
| BeO4 tetrahedra | |||||||
| Be–O2 | 1.573(9) | 1.581(9) | 1.573(10) | 1.570(9) | 1.572(10) | 1.571(10) | 1.574(9) |
| Be–O5 | 1.603(10) | 1.597(10) | 1.595(10) | 1.600(9) | 1.606(10) | 1.600(10) | 1.603(9) |
| Be–O1 | 1.598(9) | 1.594(10) | 1.613(10) | 1.612(10) | 1.611(10) | 1.607(10) | 1.598(9) |
| Be–O4 | 1.602(10) | 1.598(10) | 1.602(9) | 1.591(8) | 1.598(9) | 1.607(9) | 1.605(8) |
| <Be–O> | 1.594 | 1.592 | 1.596 | 1.593 | 1.597 | 1.596 | 1.595 |
| Volume | 2.064 | 2.058 | 2.070 | 2.060 | 2.073 | 2.074 | 2.068 |
| YO8 polyhedra | |||||||
| Y–O1 | 2.501(5) | 2.501(6) | 2.510(6) | 2.505(5) | 2.508(6) | 2.511(6) | 2.512(5) |
| Y–O4 | 2.366(5) | 2.366(6) | 2.364(6) | 2.364(5) | 2.363(5) | 2.363(5) | 2.361(5) |
| Y–O3 | 2.308(5) | 2.306(5) | 2.302(5) | 2.304(5) | 2.308(5) | 2.305(5) | 2.306(5) |
| Y–O2 | 2.537(6) | 2.537(6) | 2.527(6) | 2.535(5) | 2.532(6) | 2.531(6) | 2.534(5) |
| Y–O2 | 2.446(6) | 2.444(6) | 2.453(6) | 2.448(5) | 2.452(6) | 2.458(6) | 2.450(5) |
| Y–O3 | 2.309(6) | 2.310(6) | 2.312(5) | 2.313(5) | 2.316(6) | 2.316(6) | 2.314(5) |
| Y–O1 | 2.658(6) | 2.660(6) | 2.649(6) | 2.657(5) | 2.655(6) | 2.657(6) | 2.661(5) |
| Y–O5 | 2.374(6) | 2.380(6) | 2.376(5) | 2.382(5) | 2.373(6) | 2.377(5) | 2.380(5) |
| <Y–O> | 2.437 | 2.438 | 2.436 | 2.438 | 2.438 | 2.440 | 2.440 |
| Volume | 24.668 | 24.681 | 24.655 | 24.718 | 24.716 | 24.755 | 24.747 |
| FeO6 octahedra | |||||||
| Fe–O2 (×2) | 2.061(5) | 2.061(5) | 2.058(6) | 2.063(5) | 2.062(6) | 2.061(6) | 2.064(5) |
| Fe–O4 (×2) | 2.222(5) | 2.221(5) | 2.229(6) | 2.222(5) | 2.223(5) | 2.223(5) | 2.222(5) |
| Fe–O5 (×2) | 2.264(5) | 2.267(6) | 2.274(5) | 2.274(5) | 2.279(6) | 2.276(5) | 2.282(5) |
| <Fe–O> | 2.182 | 2.183 | 2.187 | 2.186 | 2.188 | 2.187 | 2.190 |
| Volume | 12.980 | 12.985 | 13.064 | 13.055 | 13.102 | 13.078 | 13.121 |
| H–O contact | |||||||
| H–O2 | 0.90(6) | 0.90(6) | 0.90(4) | 0.91(4) | 0.90(4) | 0.91(4) | 0.90(4) |
| T, °C | α11 | α22 | α33 | μ (α33^c) | αa | αb | αc | αβ | αV |
|---|---|---|---|---|---|---|---|---|---|
| Linear approximation | |||||||||
| (−173)–(+7) | 5(1) | 6(1) | −3(1) | 21(1) | 4(1) | 7(1) | −2(1) | 3(1) | 9(2) |
| Quadratic polynomial approximation | |||||||||
| −173 | 11(2) | −2(2) | −7(2) | 22(2) | 9(2) | −2(2) | −5(2) | 8(2) | 1(3) |
| −143 | 9(2) | 1(2) | −6(2) | 22(2) | 7(2) | 1(2) | −4(2) | 6(2) | 4(3) |
| −113 | 7(1) | 4(1) | −4(1) | 21(1) | 6(1) | 4(1) | −3(1) | 5(1) | 6(2) |
| −83 | 5(1) | 6(1) | −3(1) | 21(1) | 4(1) | 7(1) | −2(1) | 3(1) | 9(2) |
| −53 | 3(1) | 10(1) | −2(1) | 20(1) | 3(1) | 9(1) | −1(1) | 2(1) | 11(2) |
| −23 | 1(2) | 12(2) | 0(2) | 14(2) | 1(2) | 12(2) | 0(2) | 0(2) | 13(3) |
| 7 | −1(2) | 15(2) | 1(2) | 27(2) | 0(2) | 15(2) | 1(2) | −1(2) | 16(3) |
| Mineral, Formula | Dimension | αa/α11 | αb/α22 | αc/α33 | αV | Δt, °C | Ref. |
|---|---|---|---|---|---|---|---|
| Beryl, Be3Al2Si6O18 | 0D | 3/3 | 3/3 | 3/3 | 8 | 25–800 | [64] |
| Danalite, Be3Fe2+4(SiO4)3S | 3D | 5/5 | 5/5 | 5/5 | 16 | 33–1035 | [65] * |
| Euclase, BeAlSiO4(OH) | 3D | 7/4 | 10/10 | 9/11 | 24 | 25–950 | [66] * |
| Phenakite, Be2SiO4 | 3D | 5/5 | 5/5 | 6/6 | 17 | 25–690 | [67] |
| Trimerite, CaMn2(BeSiO4)3 | 3D | 9/9 | 12/12 | 9/9 | 29 | 25–800 | [68] * |
| Tugtupite, Na4BeAlSi4O12Cl | 3D | 11/11 | 11/11 | 8/8 | 31 | 33–982 | [69] * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorelova, L.; Vereshchagin, O.; Cuchet, S.; Shilovskikh, V.; Pankin, D. Low-Temperature Crystal Chemistry of Hingganite-(Y), from the Wanni Glacier, Switzerland. Minerals 2020, 10, 322. https://doi.org/10.3390/min10040322
Gorelova L, Vereshchagin O, Cuchet S, Shilovskikh V, Pankin D. Low-Temperature Crystal Chemistry of Hingganite-(Y), from the Wanni Glacier, Switzerland. Minerals. 2020; 10(4):322. https://doi.org/10.3390/min10040322
Chicago/Turabian StyleGorelova, Liudmila, Oleg Vereshchagin, Stéphane Cuchet, Vladimir Shilovskikh, and Dmitrii Pankin. 2020. "Low-Temperature Crystal Chemistry of Hingganite-(Y), from the Wanni Glacier, Switzerland" Minerals 10, no. 4: 322. https://doi.org/10.3390/min10040322
APA StyleGorelova, L., Vereshchagin, O., Cuchet, S., Shilovskikh, V., & Pankin, D. (2020). Low-Temperature Crystal Chemistry of Hingganite-(Y), from the Wanni Glacier, Switzerland. Minerals, 10(4), 322. https://doi.org/10.3390/min10040322