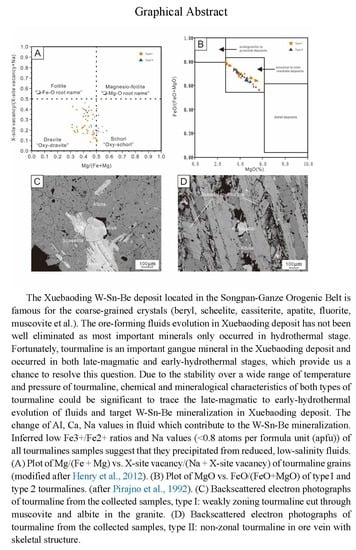
Tourmaline as a Recorder of Ore-Forming Processes in the Xuebaoding W-Sn-Be Deposit, Sichuan Province, China: Evidence from the Chemical Composition of Tourmaline
Abstract
:1. Introduction
2. Geological Setting
3. Sampling and Analytical Methods
3.1. Sampling and Petrographic Description
3.2. Analytical Methods
4. Results
4.1. Textural Features Of Tourmaline
4.2. Chemical Composition of Tourmaline
5. Discussion
5.1. Origin of Tourmaline
5.2. Chemical Evolution of Tourmaline
5.3. Implication for W-Sn-Be Mineralization
6. Conclusions
- (1)
- Three types of tourmalines are identified in the Xuebaoding deposit. Type I tourmalines are interpreted to have formed from an immiscible boron-rich magmatic-hydrothermal fluid. Type II tourmalines with skeletal texture formed earlier than type I, in a transition from late-magmatic to early-hydrothermal conditions, and type III are hydrothermal tourmalines, occurring in the mineralized veins.
- (2)
- The chemical compositions of tourmaline are buffered by the host rocks. Inferred increasing Fe3+/Fe2+ ratios and the decreasing Na values of all tourmalines studied suggest that they precipitated from oxidized, low-salinity fluids.
- (3)
- Mineralogical characteristics and chemical composition variations of tourmalines as established in this work may help in W-mineralization exploration in the larger region around Pinguw-Xuebaoding, or more generally in related geological settings.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Z.M.; Zheng, J.B.; An, W.; Li, Y.G. Geochemistry of Xuebaoding alkali granite and its ore-controlling effect. J. Ocean Univ. China 2004, 34, 874–880. [Google Scholar]
- Cao, Z.; Zheng, J.; Li, Y.; Ren, J.; Xu, S.; Wang, R.; Kabayashi, S. Geologic and geochemical features of the volatile-rich ore fluid and its tracing and dating in the Xuebaoding Beryl-Scheelite Vein Deposit, China. Sci. China Ser. D Earth Sci. 2002, 45, 719–729. [Google Scholar]
- Li, J.K.; Liu, S.B.; Wang, D.H.; Fu, X.F. Metallogenic epoch of Xuebaoding W-Sn-Be deposit in northwest Sichuan and its tectonic tracing significance. Miner. Depos. 2007, 26, 557–562, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.; Deng, J.; Li, C.; Shi, G.; Zheng, A. REE composition in scheelite and scheelite Sm-Nd dating for the Xuebaoding W-Sn-Be deposit in Sichuan. Chin. Sci. Bull. 2007, 52, 2543–2550. [Google Scholar] [CrossRef]
- Liu, Y. Mineralogical characteristics and formation mechanism of W-Sn-Be deposit in Xuebaoding, Northwest Sichuan. China Univ. Geosci. (Beijing) 2010. [Google Scholar]
- Liu, Y.; Deng, J.; Zhang, G.; Shi, G.; Yang, L.; Wang, Q. 40Ar/39Ar Dating of Xuebaoding Granite in the Songpan-Garzê Orogenic Belt, Southwest China, and its Geological Significance. Acta Geol. Sin. Engl. Ed. 2010, 84, 345–357. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Shi, G.; Sun, X.; Yang, L. Genesis of the Xuebaoding W-Sn-Be crystal deposits in southwest China: Evidence from fluid inclusions, stable isotopes and ore elements. Resour. Geol. 2012, 62, 159–173. [Google Scholar] [CrossRef]
- White, J.S.; Richards, R.P. Chinese beryl crystal mimic twinning. Rocks Miner. 1999, 74, 318. [Google Scholar] [CrossRef]
- Guo, Y.J.; Wang, R.C.; Xu, S.J. Vibrational Spectra of Beryl from Xuebaoding, Pingwu County, Sichuan Province. Geol. J. China Univ. 2000, 2. [Google Scholar]
- Jinhui, L.; Zhimin, C.; Liu, J.; Youguo, L.; Yiyun, Z.; Sancong, Y. Mineral spectroscopic studies of beryls from Xuebaoding, Sichuan. Acta Petrrol. Mineral. 2000, 4, 10. [Google Scholar]
- Zhang, D.; Peng, J.; Coulson, I.M.; Hou, L.; Li, S. Cassiterite U-Pb and muscovite 40Ar–39Ar age constraints on the timing of mineralization in the Xuebaoding Sn-W-Be deposit, western China. Ore Geol. Rev. 2014, 62, 315–322. [Google Scholar] [CrossRef]
- Zhou, K.C.; Qi, L.J.; Xiang, C.J.; Feng, Q.M.; Wan, J.S.; Hao, Q. Geologic characteristic of forming beryl gem Pingwu. Sichuan J. Miner. Petrol. 2002, 22, 1–7, (In Chinese with English Abstract). [Google Scholar]
- Balen, D.; Petrinec, Z. Contrasting tourmaline types from peraluminous granites: A case study from Moslavačka Gora (Croatia). Mineral. Petrol. 2011, 102, 117. [Google Scholar] [CrossRef]
- Benard, F.; Moutou, P.; Pichavant, M. Phase relations of tourmaline leucogranites and the significance of tourmaline in silicic magmas. J. Geol. 1985, 93, 271–291. [Google Scholar] [CrossRef]
- Cabral, A.R.; Wiedenbeck, M.; Rios, F.J.; Gomes, A.A.S.; Rocha Filho, O.G.; Jones, R.D. Talc mineralisation associated with soft hematite ore, Gongo Soco deposit, Minas Gerais, Brazil: Petrography, mineral chemistry and boron-isotope composition of tourmaline. Miner. Depos. 2012, 47, 411–424. [Google Scholar] [CrossRef]
- Dutrow, B.L.; Henry, D.J. Tourmaline: A geologic DVD. Elements 2011, 7, 301–306. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Prowatke, S.; Rossman, G.R.; London, D.; Fritz, E.A. Mn-rich tourmaline from Austria: Structure, chemistry, optical spectra, and relations to synthetic solid solutions. Am. Mineral. 2003, 88, 1369–1376. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Henry, D.J. Classification of the minerals of the tourmaline group. Eur. J. Miner. Stuttg. 1999, 11, 201–216. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Dirlam, D.M. Tourmaline the indicator mineral: From atomic arrangement to Viking navigation. Elements 2011, 7, 307–312. [Google Scholar] [CrossRef]
- Henry, D.J.; Guidotti, C.V. Tourmaline as a petrogenetic indicator mineral: An example from the staurolite-grade metapelites of NW Maine. Am. Mineral. 1985, 70, 1–15. [Google Scholar]
- Henry, D.J.; Dutrow, B.L. Metamorphic tourmaline and its petrologic applications. Rev. Mineral. Geochem. 1996, 33, 503–557. [Google Scholar]
- Henry, D.J.; Dutrow, B.L. The incorporation of fluorine in tourmaline: Internal crystallographic controls or external environmental influences? Can. Mineral. 2011, 49, 41–56. [Google Scholar] [CrossRef]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline-supergroup minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Henry, D.J.; Dutrow, B.L. Tourmaline at diagenetic to low-grade metamorphic conditions: Its petrologic applicability. Lithos 2012, 154, 16–32. [Google Scholar] [CrossRef]
- Hong, W.; Fox, N.; Cooke, D.R.; Zhang, L.; Fayek, M. B-and O-isotopic compositions of tourmaline constrain late-stage magmatic volatile exsolution in Tasmanian tin-related granite systems. Miner. Depos. 2020, 55, 63–78. [Google Scholar] [CrossRef]
- Perugini, D.; Poli, G. Tourmaline nodules from Capo Bianco aplite (Elba Island, Italy): An example of diffusion limited aggregation growth in a magmatic system. Contrib. Mineral. Petrol. 2007, 153, 493–508. [Google Scholar] [CrossRef]
- van Hinsberg, V.J.; Henry, D.J.; Marschall, H.R. Tourmaline: An ideal indicator of its host environment. Can. Mineral. 2011, 49, 1–16. [Google Scholar] [CrossRef]
- Krienitz, M.S.; Trumbull, R.B.; Hellmann, A.; Kolb, J.; Meyer, F.M.; Wiedenbeck, M. Hydrothermal gold mineralization at the Hira Buddini gold mine, India: Constraints on fluid evolution and fluid sources from boron isotopic compositions of tourmaline. Miner. Depos. 2008, 43, 421–434. [Google Scholar] [CrossRef]
- Drivenes, K.; Larsen, R.B.; Müller, A.; Sørensen, B.E.; Wiedenbeck, M.; Raanes, M.P. Late-magmatic immiscibility during batholith formation: Assessment of B isotopes and trace elements in tourmaline from the Land’s End granite, SW England. Contrib. Mineral. Petrol. 2015, 169, 56. [Google Scholar] [CrossRef]
- Huang, S.; Song, Y.; Hou, Z.; Xue, C. Chemical and stable isotopic (B, H, and O) compositions of tourmaline in the Maocaoping vein-type Cu deposit, western Yunnan, China: Constraints on fluid source and evolution. Chem. Geol. 2016, 439, 173–188. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Radvanec, M.; Nakamura, E.; Palmer, M.; Kobayashi, K.; Zhao, H.X.; Zhao, K.D. Chemical and boron isotopic variations of tourmaline in the Hnilec granite-related hydrothermal system, Slovakia: Constraints on magmatic and metamorphic fluid evolution. Lithos 2008, 106, 1–11. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Yu, J.M.; Lu, J.J. Trace and rare-earth element geochemistry in tourmaline and cassiterite from the Yunlong tin deposit, Yunnan, China: Implication for magmatic-hydrothermal fluid evolution and ore genesis. Chem. Geol. 2004, 209, 193–213. [Google Scholar] [CrossRef]
- Longfellow, K.M.; Swanson, S.E. Skeletal tourmaline, undercooling, and crystallization history of the Stone Mountain granite, Georgia, USA. Can. Mineral. 2011, 49, 341–357. [Google Scholar] [CrossRef]
- Rozendaal, A.; Bruwer, L. Tourmaline nodules: Indicators of hydrothermal alteration and Sn-Zn-(W) mineralization in the Cape Granite Suite, South Africa. J. Afr. Earth Sci. 1995, 21, 141–155. [Google Scholar] [CrossRef]
- Samson, I.M.; Sinclair, W.D. Magmatic hydrothermal fluids and the origin of quartz-tourmaline orbicules in the Seagull batholith, Yukon Territory. Can. Mineral. 1992, 30, 937–954. [Google Scholar]
- Baksheev, I.A.; Trumbull, R.B.; Popov, M.P.; Erokhin, Y.V.; Kudryavtseva, O.E.; Yapaskurt, V.O.; Kiselev, V.I. Chemical and boron isotopic composition of tourmaline from the Mariinsky emerald deposit, Central Urals, Russia. Miner. Depos. 2018, 53, 565–583. [Google Scholar] [CrossRef]
- Codeço, M.S.; Weis, P.; Trumbull, R.B.; Glodny, J.; Wiedenbeck, M.; Romer, R.L. Boron Isotope Muscovite-Tourmaline Geothermometry Indicates Fluid Cooling During Magmatic-Hydrothermal W-Sn Ore Formation. Econ. Geol. 2019, 114, 153–163. [Google Scholar] [CrossRef]
- Duan, Z.P.; Jiang, S.Y.; Su, H.M.; Zhu, X. Tourmaline as a recorder of contrasting boron source and potential tin mineralization in the Mopanshan pluton from Inner Mongolia, northeastern China. Lithos 2020, 354, 105284. [Google Scholar] [CrossRef]
- Gong, Q.J.; Yu, C.W.; Zhang, R.H. Physical chemistry study on the ore-forming process of Shizhuyuan tungsten-polymetallic deposit. Earth Sci. Front. 2004, 11, 617–625, (In Chinese with English Abstract). [Google Scholar]
- Hazarika, P.; Mishra, B.; Pruseth, K.L. Diverse tourmaline compositions from orogenic gold deposits in the Hutti-Maski Greenstone Belt, India: Implications for sources of ore-forming fluids. Econ. Geol. 2015, 110, 337–353. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Palmer, M.R.; McDonald, A.M.; Slack, J.F.; Leitch, C.H.B. Feruvite from the Sullivan Pb-Zn-Ag deposit, British Columbia. Can. Mineral. 1996, 34, 733–740. [Google Scholar]
- Ranta, J.P.; Hanski, E.; Cook, N.; Lahaye, Y. Source of boron in the Palokas gold deposit, northern Finland: Evidence from boron isotopes and major element composition of tourmaline. Miner. Depos. 2017, 52, 733–746. [Google Scholar] [CrossRef]
- Tornos, F.; Wiedenbeck, M.; Velasco, F. The boron isotope geochemistry of tourmaline-rich alteration in the IOCG systems of northern Chile: Implications for a magmatic-hydrothermal origin. Miner. Depos. 2012, 47, 483–499. [Google Scholar] [CrossRef]
- Trumbull, R.B.; Krienitz, M.S.; Gottesmann, B.; Wiedenbeck, M. Chemical and boron-isotope variations in tourmalines from an S-type granite and its source rocks: The Erongo granite and tourmalinites in the Damara Belt, Namibia. Contrib. Mineral. Petrol. 2008, 155, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Trumbull, R.B.; Garda, G.M.; Xavier, R.P.; Cavalcanti, J.A.D.; Codeço, M.S. Tourmaline in the Passagem de Mariana gold deposit (Brazil) revisited: Major-element, trace-element and B-isotope constraints on metallogenesis. Miner. Depos. 2019, 54, 395–414. [Google Scholar] [CrossRef]
- Harlaux, M.; Mercadier, J.; Marignac, C.; Villeneuve, J.; Mouthier, B.; Cuney, M. Origin of the atypical Puy-les-Vignes W breccia pipe (Massif Central, France) constrained by trace element and boron isotopic composition of tourmaline. Ore Geol. Rev. 2019, 114, 103132. [Google Scholar] [CrossRef]
- Yan, D.; Zhou, M.; Wei, G.; Liu, H.; Dong, T.; Zhang, W.; Jin, Z. Collapse of songpan-garzê orogenic belt resulted from mesozoic middle-crustal ductile channel flow: Evidences from deformation and metamorphism within sinian-paleozoic strata in hinterland of longmenshan foreland thrust belt. Earth Sci. Front. 2008, 15, 186–198. [Google Scholar] [CrossRef]
- Ye, S.; Qi, L.; Luo, Y.; Zhou, K.; Pi, J. Realationship between the rare- metal contained granitic intrusions and beryl mineralization in Pingwu, Sichuan, China. Geol. Sci. Tech. Inf. 2001, 20, 65–70, (In Chinese with English Abstract). [Google Scholar]
- Hu, Z.C.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Wu, F.; Liu, X.; Ji, W.; Wang, J.; Yang, L. Highly fractionated granites: Recognition and research. Sci. China Earth Sci. 2017, 60, 1201–1219. [Google Scholar] [CrossRef]
- Bureau of Geology and Mineral Recourses of Sichuan Province. Regional Geology of Sichuan Province; Geological Publishing House: Beijing, China, 1994; pp. 1–190. (In Chinese).
- Henry, D.J.; Sun, H.; Slack, J.F.; Dutrow, B.L. Tourmaline in meta-evaporites and highly magnesian rocks: Perspectives from Namibian tourmalinites. Eur. J. Mineral. 2008, 20, 889–904. [Google Scholar] [CrossRef]
- Wood, S.A. Theoretical prediction of speciation and solubility of beryllium in hydrothermal solution to 300 C at saturated vapor pressure: Application to bertrandite/phenakite deposits. Ore Geol. Rev. 1992, 7, 249–278. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Li, X.F.; Zhang, L.; Wang, G.; Zhang, D. The characteristics of tourmaline in Baiyanghe U-Be deposit and its implications for mineralization, Xinjiang. Acta Petrol. Sin. 2019, 35, 3429–3442, (In Chinese with English Abstract). [Google Scholar]
- Guo, J.; Yan, H.B.; Ling, M.X.; Zhang, R.Q. Chemical composition of tourmaline in the biotite granite, the Dachang district: Insights into magmatic-hydrothermal evolution. Acta Petrol. Sin. 2020, 36, 171–183, (In Chinese with English Abstract). [Google Scholar]
- Heinrich, C.A. Geochemical evolution and hydrothermal mineral deposition in Sn (-W-base metal) and other granite-related ore systems: Some conclusions from Australian examples. Short Course Handb. 1995, 23, 203–220. [Google Scholar]
- Taylor, J.R.; Wall, V.J. Cassiterite solubility, tin speciation, and transport in a magmatic aqueous phase. Econ. Geol. 1993, 88, 437–460. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The hydrothermal geochemistry of tungsten in granitoid environments: I. Relative solubilities of ferberite and scheelite as a function of T, P, pH, and m NaCl. Econ. Geol. 2000, 95, 143–182. [Google Scholar] [CrossRef] [Green Version]
- Pirajno, F.; Smithies, R.H. The FeO/(FeO + MgO) ratio of tourmaline: A useful indicator of spatial variations in granite-related hydrothermal mineral deposits. J. Geochem. Explor. 1992, 42, 371–381. [Google Scholar] [CrossRef]








| Sample NO. | XBD 11c | XBD 11d | XBD 11e | XBD 11f | XBD 11g | XBD 21a | XBD 21b | XBD 21c | XBD 21d | XBD 21f | XBD 21h | XBD 21i | XBD 21j | XBD 21k | XBD 21l | XBD 21m | XBD 21o | XBD 21p | XBD 21q | XBD 21r | XBD 21s | XBD 21t | XBD 22a |
| SiO2 | 35.64 | 35.9 | 35.24 | 36.27 | 35.48 | 36.92 | 36.98 | 36.63 | 37.1 | 37 | 37.09 | 36.14 | 36.67 | 35.53 | 35.83 | 37.24 | 36.92 | 36.53 | 36.78 | 36.58 | 36.53 | 35.99 | 36.19 |
| TiO2 | 0.63 | 1.12 | 0.72 | 0.97 | 1.27 | 0.35 | 0.43 | 0.29 | 0.42 | 0.41 | 0.78 | 0.72 | 0.4 | 0.82 | 0.76 | 0.2 | 0.69 | 0.5 | 0.42 | 0.33 | 0.4 | 0.87 | 0.75 |
| Al2O3 | 32.36 | 32.52 | 32.27 | 32.34 | 32.13 | 34.07 | 33.15 | 33.88 | 31.52 | 31.19 | 32.16 | 33.08 | 33.42 | 32.37 | 32.32 | 33.88 | 32.62 | 33.35 | 33.5 | 31.59 | 32.43 | 33.39 | 32.29 |
| MgO | 4.6 | 3.78 | 4.64 | 5.68 | 4.07 | 3.07 | 3.14 | 2.76 | 5.03 | 4.93 | 4.19 | 3.67 | 3.42 | 3.9 | 3.7 | 4.07 | 4.13 | 3.83 | 3.79 | 4.89 | 4.38 | 2.84 | 3.04 |
| MnO | 0.4 | 0.38 | 0.39 | 0.3 | 0.4 | 0.4 | 0.45 | 0.46 | 0.2 | 0.17 | 0.37 | 0.33 | 0.31 | 0.34 | 0.41 | 0.09 | 0.42 | 0.35 | 0.41 | 0.15 | 0.19 | 0.44 | 0.43 |
| FeO | 9.19 | 9 | 8.91 | 7.4 | 9.62 | 10.35 | 10.3 | 10.72 | 9.95 | 10.03 | 9.4 | 10 | 9.05 | 9.47 | 9.6 | 8.16 | 8.93 | 9.07 | 9.47 | 9.57 | 10.3 | 10.42 | 10.07 |
| Cr2O3 | 0 | 0 | 0 | 0.02 | 0 | 0.01 | 0.01 | 0.03 | 0 | 0.02 | 0 | 0.02 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0.02 | 0.03 |
| CaO | 1.08 | 1.15 | 1.16 | 1.25 | 1.15 | 0.37 | 0.65 | 0.37 | 0.76 | 0.72 | 1.07 | 0.92 | 0.44 | 1.18 | 0.98 | 0.06 | 1.15 | 0.79 | 0.55 | 0.7 | 1.01 | 0.92 | 0.82 |
| Na2O | 2.15 | 2.14 | 2.14 | 2.07 | 2.07 | 1.84 | 2.34 | 1.73 | 2.4 | 2.4 | 2.04 | 2.28 | 1.77 | 2.01 | 2.01 | 2.08 | 2.39 | 1.98 | 2.04 | 2.5 | 2.18 | 2.18 | 2.18 |
| K2O | 0.04 | 0.03 | 0.02 | 0.03 | 0.04 | 0.02 | 0.07 | 0.02 | 0.05 | 0.05 | 0.04 | 0.05 | 0.02 | 0.06 | 0.03 | 0.03 | 0.06 | 0.04 | 0.05 | 0.05 | 0.04 | 0.06 | 0.04 |
| Total | 86.1 | 86.01 | 85.49 | 86.33 | 86.23 | 87.39 | 87.51 | 86.9 | 87.43 | 86.92 | 87.13 | 87.21 | 85.55 | 85.68 | 85.62 | 85.8 | 87.31 | 86.42 | 87.02 | 86.36 | 87.47 | 87.12 | 85.84 |
| Si | 5.66 | 5.74 | 5.63 | 5.7 | 5.66 | 5.84 | 5.82 | 5.85 | 5.8 | 5.82 | 5.85 | 5.69 | 5.9 | 5.7 | 5.76 | 5.92 | 5.78 | 5.8 | 5.81 | 5.77 | 5.73 | 5.71 | 5.82 |
| Al(total) | 6.05 | 6.11 | 6.07 | 5.98 | 6.03 | 6.34 | 6.14 | 6.36 | 5.79 | 5.77 | 5.97 | 6.13 | 6.33 | 6.11 | 6.11 | 6.34 | 6.01 | 6.23 | 6.22 | 5.86 | 5.98 | 6.23 | 6.11 |
| Al(T) | 0.05 | 0.11 | 0.07 | 0 | 0.03 | 0.16 | 0.14 | 0.15 | 0 | 0 | 0 | 0.13 | 0.1 | 0.11 | 0.11 | 0.08 | 0.01 | 0.2 | 0.19 | 0 | 0 | 0.23 | 0.11 |
| Al(Z) | 6 | 6 | 6 | 5.98 | 6 | 6 | 6 | 6 | 5.79 | 5.77 | 5.97 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5.86 | 5.98 | 6 | 6 |
| Al(Y) | 0 | 0 | 0 | 0 | 0 | 0.18 | 0 | 0.21 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0 | 0.26 | 0 | 0.03 | 0.03 | 0 | 0 | 0 | 0 |
| Ti | 0.08 | 0.13 | 0.09 | 0.11 | 0.15 | 0.04 | 0.05 | 0.03 | 0.05 | 0.05 | 0.09 | 0.09 | 0.05 | 0.1 | 0.09 | 0.02 | 0.08 | 0.06 | 0.05 | 0.04 | 0.05 | 0.1 | 0.09 |
| Mg | 1.1 | 0.91 | 1.11 | 1.34 | 0.97 | 0.73 | 0.74 | 0.66 | 1.18 | 1.16 | 0.99 | 0.87 | 0.83 | 0.94 | 0.89 | 0.97 | 0.97 | 0.91 | 0.9 | 1.16 | 1.03 | 0.68 | 0.73 |
| Mn | 0.05 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.06 | 0.06 | 0.03 | 0.02 | 0.05 | 0.04 | 0.04 | 0.05 | 0.06 | 0.01 | 0.06 | 0.05 | 0.06 | 0.02 | 0.03 | 0.06 | 0.06 |
| Fe(total) | 1.22 | 1.2 | 1.19 | 0.97 | 1.28 | 1.36 | 1.35 | 1.43 | 1.3 | 1.31 | 1.24 | 1.31 | 1.21 | 1.27 | 1.29 | 1.08 | 1.17 | 1.2 | 1.25 | 1.26 | 1.35 | 1.38 | 1.35 |
| Cr | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ca | 0.18 | 0.2 | 0.2 | 0.21 | 0.2 | 0.06 | 0.11 | 0.06 | 0.13 | 0.12 | 0.18 | 0.15 | 0.08 | 0.2 | 0.17 | 0.01 | 0.19 | 0.13 | 0.09 | 0.12 | 0.17 | 0.16 | 0.14 |
| Na | 0.66 | 0.66 | 0.66 | 0.63 | 0.64 | 0.56 | 0.71 | 0.53 | 0.72 | 0.73 | 0.62 | 0.69 | 0.55 | 0.62 | 0.62 | 0.64 | 0.72 | 0.61 | 0.62 | 0.76 | 0.66 | 0.67 | 0.68 |
| K | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0 | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| X-site vacancy | 0.15 | 0.14 | 0.13 | 0.15 | 0.15 | 0.37 | 0.16 | 0.4 | 0.14 | 0.14 | 0.19 | 0.14 | 0.37 | 0.16 | 0.2 | 0.35 | 0.07 | 0.25 | 0.27 | 0.11 | 0.16 | 0.16 | 0.17 |
| Species name | Dravite | Dravite | Dravite | Schorl | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite |
| Sample NO. | XBD 22b | XBD 22c | XBD 22e | XBD 22f | XBD 22g | XBD 22h | XBD 22e | XBD 22f | XBD 22g | XBD 22h | XBD 22i | XBD 22j | XBD 22k | XBD 34a | XBD 34b | XBD 34c | XBD 34d | XBD 34e | XBD 34f | XBD 34g | XBD 34h | XBD 34i | XBD 34 |
| SiO2 | 37.15 | 36.85 | 35.4 | 37.31 | 36.87 | 37.21 | 35.4 | 37.31 | 36.87 | 37.21 | 36.02 | 37.24 | 37.31 | 36.88 | 36.09 | 36.24 | 36.14 | 36.53 | 36.73 | 36.77 | 36.67 | 36.49 | 36.79 |
| TiO2 | 0.23 | 0.28 | 0.73 | 0.3 | 0.37 | 0.3 | 0.73 | 0.3 | 0.37 | 0.3 | 0.29 | 0.27 | 0.24 | 0.78 | 0.72 | 0.41 | 0.78 | 0.75 | 0.91 | 0.73 | 0.54 | 0.5 | 0.84 |
| Al2O3 | 33.27 | 33.46 | 31.93 | 33.82 | 33.35 | 33.16 | 31.93 | 33.82 | 33.35 | 33.16 | 32.64 | 33.94 | 34.25 | 32.85 | 32.51 | 32.43 | 32.35 | 32.43 | 32.21 | 30.15 | 30.5 | 30.67 | 30.31 |
| MgO | 4.51 | 3.43 | 2.98 | 3.88 | 3.8 | 3.84 | 2.98 | 3.88 | 3.8 | 3.84 | 3.71 | 3.94 | 3.7 | 2.71 | 2.81 | 2.71 | 3.59 | 3.67 | 4.66 | 5.32 | 5.33 | 5.43 | 5.52 |
| MnO | 0.31 | 0.35 | 0.5 | 0.38 | 0.3 | 0.44 | 0.5 | 0.38 | 0.3 | 0.44 | 0.69 | 0.36 | 0.32 | 0.46 | 0.42 | 0.43 | 0.41 | 0.4 | 0.32 | 0.12 | 0.15 | 0.11 | 0.11 |
| FeO | 8.01 | 9.34 | 10.08 | 8.14 | 8.85 | 8.8 | 10.08 | 8.14 | 8.85 | 8.8 | 9.17 | 8.94 | 9.3 | 10.72 | 10 | 10.74 | 10.15 | 10.02 | 8.3 | 9.84 | 9.47 | 9.84 | 9.77 |
| Cr2O3 | 0.05 | 0.02 | 0 | 0.01 | 0.05 | 0.1 | 0 | 0.01 | 0.05 | 0.1 | 0.07 | 0.03 | 0.02 | 0 | 0 | 0.01 | 0.01 | 0.05 | 0.01 | 0 | 0.02 | 0.01 | 0 |
| CaO | 0.34 | 0.55 | 0.87 | 0.37 | 0.39 | 0.54 | 0.87 | 0.37 | 0.39 | 0.54 | 0.5 | 0.36 | 0.39 | 0.78 | 0.92 | 0.8 | 1.01 | 1.06 | 1.2 | 1.53 | 1.34 | 1.48 | 1.63 |
| Na2O | 1.8 | 1.95 | 2.04 | 1.85 | 1.99 | 1.93 | 2.04 | 1.85 | 1.99 | 1.93 | 1.77 | 1.76 | 1.84 | 2.09 | 2.19 | 2.13 | 2.06 | 2.03 | 2.12 | 2.08 | 2.23 | 2.14 | 2.15 |
| K2O | 0.03 | 0.01 | 0.03 | 0.03 | 0.04 | 0.05 | 0.03 | 0.03 | 0.04 | 0.05 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.05 | 0.06 | 0.04 | 0.05 | 0.03 | 0.04 | 0.03 |
| Total | 85.71 | 86.23 | 84.55 | 86.09 | 86.01 | 86.37 | 84.55 | 86.09 | 86.01 | 86.37 | 84.9 | 86.86 | 87.39 | 87.29 | 85.69 | 85.92 | 86.55 | 86.99 | 86.51 | 86.58 | 86.28 | 86.72 | 87.15 |
| Si | 5.92 | 5.88 | 5.79 | 5.94 | 5.88 | 5.91 | 5.79 | 5.94 | 5.88 | 5.91 | 5.84 | 5.89 | 5.87 | 5.86 | 5.82 | 5.84 | 5.76 | 5.79 | 5.8 | 5.82 | 5.8 | 5.75 | 5.78 |
| Al(total) | 6.24 | 6.28 | 6.14 | 6.33 | 6.26 | 6.2 | 6.14 | 6.33 | 6.26 | 6.2 | 6.23 | 6.31 | 6.34 | 6.14 | 6.17 | 6.15 | 6.07 | 6.05 | 5.99 | 5.61 | 5.68 | 5.69 | 5.6 |
| Al(T) | 0.08 | 0.12 | 0.14 | 0.06 | 0.12 | 0.09 | 0.14 | 0.06 | 0.12 | 0.09 | 0.16 | 0.11 | 0.13 | 0.14 | 0.17 | 0.15 | 0.07 | 0.05 | 0 | 0 | 0 | 0 | 0 |
| Al(Z) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5.99 | 5.61 | 5.68 | 5.69 | 5.6 |
| Al(Y) | 0.16 | 0.16 | 0 | 0.27 | 0.14 | 0.12 | 0 | 0.27 | 0.14 | 0.12 | 0.07 | 0.2 | 0.21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ti | 0.03 | 0.03 | 0.09 | 0.04 | 0.04 | 0.04 | 0.09 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 | 0.09 | 0.09 | 0.05 | 0.09 | 0.09 | 0.11 | 0.09 | 0.06 | 0.06 | 0.1 |
| Mg | 1.08 | 0.82 | 0.73 | 0.93 | 0.91 | 0.92 | 0.73 | 0.93 | 0.91 | 0.92 | 0.9 | 0.94 | 0.87 | 0.65 | 0.68 | 0.66 | 0.86 | 0.87 | 1.1 | 1.26 | 1.27 | 1.28 | 1.3 |
| Mn | 0.04 | 0.05 | 0.07 | 0.05 | 0.04 | 0.06 | 0.07 | 0.05 | 0.04 | 0.06 | 0.1 | 0.05 | 0.04 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 |
| Fe(total) | 1.06 | 1.24 | 1.37 | 1.08 | 1.18 | 1.17 | 1.37 | 1.08 | 1.18 | 1.17 | 1.24 | 1.18 | 1.22 | 1.42 | 1.34 | 1.44 | 1.35 | 1.32 | 1.09 | 1.3 | 1.25 | 1.29 | 1.28 |
| Cr | 0.01 | 0 | 0 | 0 | 0.01 | 0.01 | 0 | 0 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 |
| Ca | 0.06 | 0.09 | 0.15 | 0.06 | 0.07 | 0.09 | 0.15 | 0.06 | 0.07 | 0.09 | 0.09 | 0.06 | 0.07 | 0.13 | 0.16 | 0.14 | 0.17 | 0.18 | 0.2 | 0.26 | 0.23 | 0.25 | 0.27 |
| Na | 0.56 | 0.6 | 0.65 | 0.57 | 0.61 | 0.59 | 0.65 | 0.57 | 0.61 | 0.59 | 0.56 | 0.54 | 0.56 | 0.64 | 0.68 | 0.66 | 0.64 | 0.62 | 0.65 | 0.64 | 0.68 | 0.65 | 0.65 |
| K | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| X-site vacancy | 0.38 | 0.3 | 0.2 | 0.36 | 0.31 | 0.3 | 0.2 | 0.36 | 0.31 | 0.3 | 0.35 | 0.4 | 0.37 | 0.22 | 0.15 | 0.19 | 0.18 | 0.19 | 0.14 | 0.09 | 0.08 | 0.09 | 0.07 |
| Species name | Schorl | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Schorl | Dravite | Schorl | Dravite | Schorl |
| Sample NO. | TUR 72a | TUR 72b | TUR 72c | TUR 72d | TUR 72e | TUR 72f | TUR 74a | TUR 74b | TUR 74c | TUR 74d | TUR 74e | TUR 74f | TUR 74g | TUR 74h | TUR 74i | TUR 74j | TUR 74k |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 36.39 | 36.55 | 36.83 | 36.22 | 36.46 | 36.53 | 36.47 | 36.47 | 36.38 | 36.52 | 35.69 | 36.70 | 35.80 | 36.92 | 36.72 | 36.58 | 36.25 |
| TiO2 | 0.36 | 0.70 | 0.53 | 0.64 | 0.80 | 0.47 | 0.33 | 0.22 | 0.16 | 0.21 | 0.22 | 0.33 | 0.41 | 0.27 | 0.18 | 0.31 | 0.48 |
| Al2O3 | 33.67 | 32.73 | 33.32 | 32.18 | 32.16 | 32.68 | 33.67 | 33.99 | 34.20 | 33.96 | 33.49 | 33.49 | 32.74 | 33.82 | 33.99 | 33.62 | 32.65 |
| MgO | 3.47 | 4.56 | 4.23 | 4.61 | 4.23 | 4.60 | 3.86 | 4.02 | 3.69 | 3.50 | 3.69 | 4.40 | 4.85 | 4.54 | 3.74 | 3.74 | 4.72 |
| MnO | 0.16 | 0.15 | 0.12 | 0.13 | 0.15 | 0.15 | 0.14 | 0.14 | 0.13 | 0.14 | 0.09 | 0.11 | 0.15 | 0.14 | 0.11 | 0.10 | 0.13 |
| FeO | 10.03 | 8.41 | 8.93 | 9.09 | 8.83 | 8.22 | 9.22 | 9.20 | 9.48 | 9.65 | 8.85 | 8.89 | 8.14 | 8.59 | 9.74 | 9.32 | 8.10 |
| Cr2O3 | 1.10 | 0.03 | 0.04 | 0.01 | 0.00 | 0.05 | 0.02 | 0.06 | 0.00 | 0.00 | 0.01 | 0.00 | 0.07 | 0.02 | 0.03 | 0.00 | 0.01 |
| CaO | 0.33 | 1.01 | 0.81 | 0.98 | 1.01 | 0.99 | 0.47 | 0.27 | 0.29 | 0.37 | 0.38 | 0.42 | 0.71 | 0.40 | 0.35 | 0.38 | 0.96 |
| Na2O | 1.48 | 2.05 | 2.12 | 2.17 | 2.03 | 2.06 | 1.87 | 1.91 | 1.64 | 1.78 | 1.88 | 2.10 | 1.84 | 2.06 | 1.97 | 2.00 | 2.18 |
| K2O | 0.02 | 0.01 | 0.04 | 0.04 | 0.03 | 0.02 | 0.03 | 0.00 | 0.03 | 0.01 | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 | 0.03 | 0.02 |
| Total | 87.00 | 86.19 | 86.96 | 86.05 | 85.69 | 85.75 | 86.07 | 86.27 | 85.98 | 86.14 | 84.31 | 86.46 | 84.74 | 86.77 | 86.84 | 86.07 | 85.48 |
| Si | 5.80 | 5.79 | 5.79 | 5.75 | 5.83 | 5.81 | 5.81 | 5.79 | 5.82 | 5.83 | 5.80 | 5.80 | 5.76 | 5.80 | 5.80 | 5.83 | 5.77 |
| Al(total) | 6.32 | 6.10 | 6.17 | 6.01 | 6.05 | 6.11 | 6.32 | 6.35 | 6.43 | 6.38 | 6.40 | 6.22 | 6.20 | 6.25 | 6.32 | 6.30 | 6.11 |
| Al(T) | 0.20 | 0.10 | 0.17 | 0.01 | 0.05 | 0.11 | 0.19 | 0.21 | 0.18 | 0.17 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.17 | 0.11 |
| Al(Z) | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Al(Y) | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.14 | 0.25 | 0.22 | 0.20 | 0.02 | 0.00 | 0.06 | 0.13 | 0.13 | 0.00 |
| Ti | 0.04 | 0.08 | 0.06 | 0.08 | 0.10 | 0.06 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 0.04 | 0.05 | 0.03 | 0.02 | 0.04 | 0.06 |
| Mg | 0.83 | 1.08 | 1.00 | 1.10 | 1.01 | 1.10 | 0.92 | 0.96 | 0.89 | 0.84 | 0.90 | 1.04 | 1.17 | 1.07 | 0.89 | 0.89 | 1.13 |
| Mn | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 |
| Fe(total) | 1.33 | 1.11 | 1.17 | 1.20 | 1.18 | 1.09 | 1.22 | 1.22 | 1.26 | 1.28 | 1.20 | 1.17 | 1.09 | 1.12 | 1.28 | 1.24 | 1.07 |
| Cr | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.06 | 0.17 | 0.14 | 0.17 | 0.17 | 0.17 | 0.08 | 0.05 | 0.05 | 0.06 | 0.07 | 0.07 | 0.12 | 0.07 | 0.06 | 0.06 | 0.16 |
| Na | 0.46 | 0.63 | 0.64 | 0.67 | 0.63 | 0.63 | 0.58 | 0.59 | 0.51 | 0.55 | 0.59 | 0.64 | 0.57 | 0.63 | 0.60 | 0.62 | 0.67 |
| K | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| X-site vacancy | 0.48 | 0.20 | 0.21 | 0.16 | 0.19 | 0.19 | 0.34 | 0.37 | 0.44 | 0.38 | 0.34 | 0.28 | 0.30 | 0.30 | 0.34 | 0.31 | 0.16 |
| Species name | Dravite | Dravite | Dravite | Dravite | Dravite | Schorl | Dravite | Dravite | Dravite | Dravite | Dravite | Dravite | Schorl | Dravite | Dravite | Dravite | Schorl |
| Sample NO. | XBDTur2 | XBDTur3 | XBDTur4 | XBDTur5 | XBDTur6 | XBDTur7 | XBDTur8 | XBDTur11 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 37.90 | 38.03 | 37.93 | 37.84 | 38.20 | 37.28 | 37.23 | 37.91 |
| TiO2 | 0.33 | 0.28 | 0.04 | 0.93 | 0.16 | 0.45 | 0.96 | 0.97 |
| Al2O3 | 32.99 | 33.05 | 32.77 | 32.38 | 32.91 | 30.18 | 30.92 | 31.49 |
| MgO | 3.67 | 3.72 | 3.67 | 4.45 | 3.94 | 4.73 | 4.38 | 4.46 |
| MnO | 0.51 | 0.31 | 0.45 | 0.16 | 0.37 | 0.26 | 0.35 | 0.36 |
| FeO | 8.83 | 8.93 | 8.60 | 8.52 | 9.32 | 7.94 | 8.25 | 8.41 |
| Cr2O3 | 0.00 | 0.15 | 0.06 | 0.00 | 0.01 | 0.08 | 0.03 | 0.03 |
| CaO | 0.24 | 0.26 | 0.31 | 1.18 | 0.17 | 1.09 | 1.31 | 1.34 |
| Na2O | 1.30 | 1.66 | 1.48 | 1.66 | 1.70 | 1.94 | 1.80 | 1.83 |
| K2O | 0.05 | 0.02 | 0.03 | 0.06 | 0.04 | 0.12 | 0.04 | 0.04 |
| Total | 85.84 | 86.40 | 85.33 | 87.17 | 86.82 | 84.06 | 85.28 | 86.85 |
| Si | 5.92 | 5.93 | 5.99 | 5.92 | 5.91 | 6.08 | 5.97 | 5.97 |
| Al(total) | 5.85 | 5.84 | 5.87 | 5.74 | 5.78 | 5.58 | 5.62 | 5.62 |
| Al(T) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al(Z) | 5.85 | 5.84 | 5.87 | 5.74 | 5.78 | 5.58 | 5.62 | 5.62 |
| Al(Y) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ti | 0.07 | 0.06 | 0.01 | 0.19 | 0.03 | 0.09 | 0.20 | 0.20 |
| Mg | 0.74 | 0.75 | 0.74 | 0.89 | 0.78 | 0.99 | 0.90 | 0.90 |
| Mn | 0.13 | 0.08 | 0.12 | 0.04 | 0.10 | 0.07 | 0.09 | 0.09 |
| Fe(total) | 2.30 | 2.32 | 2.26 | 2.22 | 2.40 | 2.16 | 2.21 | 2.21 |
| Cr | 0.00 | 0.03 | 0.01 | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 |
| Ca | 0.06 | 0.06 | 0.07 | 0.28 | 0.04 | 0.27 | 0.32 | 0.32 |
| Na | 0.32 | 0.41 | 0.37 | 0.41 | 0.42 | 0.50 | 0.46 | 0.46 |
| K | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 |
| X-site vacancy | 0.60 | 0.52 | 0.55 | 0.29 | 0.53 | 0.19 | 0.21 | 0.21 |
| Species name | Foitite | Foitite | Foitite | Schorl | Foitite | Schorl | Schorl | Schorl |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Raschke, M.B.; Liu, Y. Tourmaline as a Recorder of Ore-Forming Processes in the Xuebaoding W-Sn-Be Deposit, Sichuan Province, China: Evidence from the Chemical Composition of Tourmaline. Minerals 2020, 10, 438. https://doi.org/10.3390/min10050438
Zhu X, Raschke MB, Liu Y. Tourmaline as a Recorder of Ore-Forming Processes in the Xuebaoding W-Sn-Be Deposit, Sichuan Province, China: Evidence from the Chemical Composition of Tourmaline. Minerals. 2020; 10(5):438. https://doi.org/10.3390/min10050438
Chicago/Turabian StyleZhu, Xinxiang, Markus B. Raschke, and Yan Liu. 2020. "Tourmaline as a Recorder of Ore-Forming Processes in the Xuebaoding W-Sn-Be Deposit, Sichuan Province, China: Evidence from the Chemical Composition of Tourmaline" Minerals 10, no. 5: 438. https://doi.org/10.3390/min10050438
APA StyleZhu, X., Raschke, M. B., & Liu, Y. (2020). Tourmaline as a Recorder of Ore-Forming Processes in the Xuebaoding W-Sn-Be Deposit, Sichuan Province, China: Evidence from the Chemical Composition of Tourmaline. Minerals, 10(5), 438. https://doi.org/10.3390/min10050438