Clean and Feasible Utilization of High Silica Fluorspar Powder via Reverse Flotation: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Flotation Experiments
2.2. Measurement of Zeta Potential
2.3. FTIR Spectral Analysis
2.4. Batch Flotation Experiment
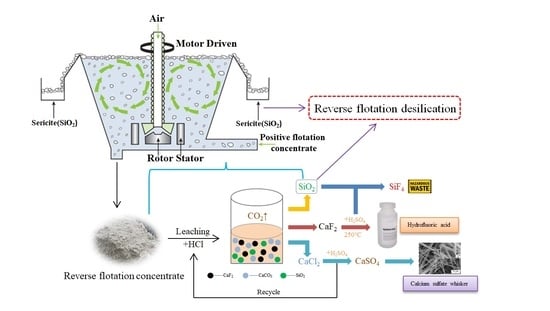
2.5. Pilot Test
3. Results and Discussion
3.1. Micro-Flotation of Pure Minerals
3.2. Zeta Potential (ZP)
3.3. Solution Chemistry Analysis
3.4. FTIR Results
3.5. Batch Flotation Experiment Results
3.6. Pilot Test Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P.B. Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 2021, 290, 102382. [Google Scholar] [CrossRef]
- Shuai, F.; Xu, L.; Wu, H.; Jia, T.; Lu, Z.; Wei, S.; Hu, Y. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Kou, J.; Tao, D.; Xu, G. Fatty acid collectors for phosphate flotation and their adsorption behavior using QCM-D. Int. J. Miner. Process. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Yang, F.; Sun, W. The flotation separation of scheelite from calcite using a quaternary ammonium salt as collector. Miner. Eng. 2011, 24, 82–84. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, M.; Xia, L.; Fu, W.; Zhou, W.; Yang, S. The utilization of citric acid as a depressant for the flotation separation of barite from fluorite. Miner. Eng. 2020, 156, 106491. [Google Scholar] [CrossRef]
- Filippov, L.; Filippova, I.; Severov, V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Ma, X.; Marques, M.; Gontijo, C. Comparative studies of reverse cationic/anionic flotation of Vale iron ore. Int. J. Miner. Process. 2011, 100, 179–183. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Liu, H.; Khoso, S.A.; Sun, W.; Zhu, Y.; Han, H.; Hu, Y.; Kang, J.; Meng, X.; Zhang, Q. A novel method for desulfurization and purification of fluorite concentrate using acid leaching and reverse flotation of sulfide. J. Clean. Prod. 2019, 209, 1006–1015. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Hu, Y.; Tang, H.; Yin, Z.; Guan, Q.; Gao, J. Investigation of two-stage depressing by using hydrophilic polymer to improve the process of fluorite flotation. J. Clean. Prod. 2018, 193, 228–235. [Google Scholar] [CrossRef]
- Mowla, D.; Karimi, G.; Ostadnezhad, K. Removal of hematite from silica sand ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Mohammadkhani, M.; Noaparast, M.; Shafaei, S.; Amini, A.; Amini, E.; Abdollahi, H. Double reverse flotation of a very low grade sedimentary phosphate rock, rich in carbonate and silicate. Int. J. Miner. Process. 2011, 100, 157–165. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Huang, L.; Huang, Z.; Li, H. The anionic flotation of fluorite from barite using gelatinized starch as the depressant. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 597, 124794. [Google Scholar] [CrossRef]
- Lin, L.; Jiong-tian, L.; Yong-tian, W.; Yi-jun, C.; Hai-jun, Z.; He-sheng, Y. Experimental research on anionic reverse flotation of hematite with a flotation column. Procedia Earth Planet. Sci. 2009, 1, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Hu, Y.; Zhao, S.; Xia, L. The role of cationic polyacrylamide in the reverse flotation of diasporic bauxite. Miner. Eng. 2007, 20, 1191–1199. [Google Scholar] [CrossRef]
- Xu, L.; Longhua, X.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic–nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Sahoo, H.; Rath, S.S.; Das, B.; Mishra, B.K. Flotation of quartz using ionic liquid collectors with different functional groups and varying chain lengths. Miner. Eng. 2016, 95, 107–112. [Google Scholar] [CrossRef]
- Lima, N.P.; Valadão, G.E.; Peres, A.E. Effect of amine and starch dosages on the reverse cationic flotation of an iron ore. Miner. Eng. 2013, 45, 180–184. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Y.; Yang, S.; Fu, W.; Chen, X. Exploration of a novel depressant polyepoxysuccinic acid for the flotation sep-aration of pentlandite from lizardite slimes. Appl. Clay Sci. 2020, 105939. [Google Scholar] [CrossRef]
- Gan, W.; Crozier, B.; Liu, Q. Effect of citric acid on inhibiting hexadecane–quartz coagulation in aqueous solutions containing Ca2+, Mg2+ and Fe3+ ions. Int. J. Miner. Process. 2009, 92, 84–91. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, Y.; Zhu, Y.; Hu, Y.; Sun, W. Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule. Minerals 2016, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; He, J.; Zhang, C.; Zhang, C.; Sun, W.; Zhao, D.; Chen, P.; Han, H.; Gao, Z.; Liu, R.; et al. Insights into the activation mechanism of calcium ions on the sericite surface: A combined experimental and computational study. Appl. Surf. Sci. 2018, 427, 162–168. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y.-H.; Liu, X.-W. Anisotropic surface broken bond properties and wettability of calcite and fluorite crystals. Trans. Nonferrous Met. Soc. China 2012, 22, 1203–1208. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D. Solution Chemistry: Minerals and Reagents; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 0080465072. [Google Scholar]
- Al-Khaldi, M.; Nasr-El-Din, H.; Mehta, S.; Al-Aamri, A. Reaction of citric acid with calcite. Chem. Eng. Sci. 2007, 62, 5880–5896. [Google Scholar] [CrossRef] [Green Version]
- Nagy, N.M.; Konya, J.; Kónya, I. Ion-exchange processes of lead and cobalt ions on the surface of calcium-montmorillonite in the presence of complex-forming agents II. The effect of DTPA, tartaric acid and citric acid on the sorption of lead ions on calcium-montmorillonite. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 137, 243–252. [Google Scholar] [CrossRef]
- Tian, J.; Gao, H.; Guan, J.; Ren, Z. Modified floc-flotation in fine sericite flotation using polymethylhydrosiloxane. Sep. Purif. Technol. 2017, 174, 439–444. [Google Scholar] [CrossRef]
- Carrara, C.; Irusta, S.; Lombardo, E.; Cornaglia, L. Study of the Co-VPO interaction in promoted n-butane oxidation catalysts. Appl. Catal. A Gen. 2001, 217, 275–286. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Adsorption of cyanide ion on pressmud surface: A modeling approach. Chem. Eng. J. 2012, 191, 548–556. [Google Scholar] [CrossRef]
- Warren, L.J. Determination of the contributions of true flotation and entrainment in batch flotation tests. Int. J. Miner. Process. 1985, 14, 33–44. [Google Scholar] [CrossRef]

















| Sample | Condition |
|---|---|
| 1 | Fluorite concentrate |
| 2 | Direct flotation (unwashed ore) + SS (200 g/t) + NaOl (50 g/t) |
| 3 | Direct flotation (washed ore) + SS (200 g/t) + NaOl (50 g/t) |
| 4 | Reverse flotation (unwashed ore) + CA (5 kg/t) + DDA (50 g/t) |
| 5 | Reverse flotation (washed ore) + CA (5 kg/t) + DDA (50 g/t) |
| Consumption | Unit Price (USD/t) | 1# (t/d) | 2# (t/d) | ∆ Profit (USD/d) |
|---|---|---|---|---|
| Fluorite powder (95 ≤ CaF2 < 97%) | 154.04 | 614.72 | / | +17,583.32 |
| Fluorite powder (CaF2 ≥ 97%) | 190.71 | / | 588.72 | |
| Diluted hydrochloric | 58.68 | 73.77 | 49.18 | +1442.94 |
| Citric acid | 440.1 | / | 10 | −4401 |
| Dodecylamine | 3374.1 | / | 0.1 | −337.41 |
| Waste water treatment cost | 2.934 | 1803.59 | 1727.30 | +76.29 |
| Power | 0.3814 | 234.45 | 224.53 | +9.92 |
| Total | / | / | / | +14,374.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Gao, J.; Hu, Y.; Sun, W.; Lv, F.; Liu, Z. Clean and Feasible Utilization of High Silica Fluorspar Powder via Reverse Flotation: A Pilot Study. Minerals 2021, 11, 555. https://doi.org/10.3390/min11060555
Zhang C, Gao J, Hu Y, Sun W, Lv F, Liu Z. Clean and Feasible Utilization of High Silica Fluorspar Powder via Reverse Flotation: A Pilot Study. Minerals. 2021; 11(6):555. https://doi.org/10.3390/min11060555
Chicago/Turabian StyleZhang, Chenhu, Jiande Gao, Yuehua Hu, Wei Sun, Fei Lv, and Zhenjun Liu. 2021. "Clean and Feasible Utilization of High Silica Fluorspar Powder via Reverse Flotation: A Pilot Study" Minerals 11, no. 6: 555. https://doi.org/10.3390/min11060555
APA StyleZhang, C., Gao, J., Hu, Y., Sun, W., Lv, F., & Liu, Z. (2021). Clean and Feasible Utilization of High Silica Fluorspar Powder via Reverse Flotation: A Pilot Study. Minerals, 11(6), 555. https://doi.org/10.3390/min11060555