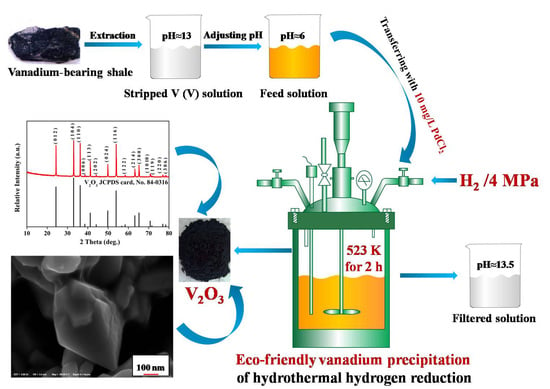
A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology
Abstract
:1. Introduction
2. Thermodynamic Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Procedures and Characterizations
3.2.1. Preparation of Stripped V (V) Solution
3.2.2. Vanadium Precipitation of Hydrothermal Hydrogen Reduction
3.2.3. Characterizations
3.3. Data Treatment
4. Results and Discussion
4.1. Effect of Initial Solution pH on Vanadium Precipitation
4.2 Effect of Reaction Time on Vanadium Precipitation
4.3. Effect of Reaction Temperature on Vanadium Precipitation
4.4. H2 Partial Pressureon Vanadium Precipitation
4.5. Phase Transformation Mechanismof the Vanadium Precipitation
5. Conclusions
- (1)
- According to the thermodynamic analysis, it is feasible for V (V) in solution to be reduced to V (III) in forms of V2O3 by H2 gas under a suitable range of solution pH value. In addition, the electromotive force (ΔE) reflects the thermodynamic drive force of the overall reduction decreases as the pH value of the vanadium solution increases, which indicates the hydrogen reduction tends to be more completely reactive at a lower pH value under the pH range in which V2O3 can exist stably.
- (2)
- The V2O3 products of 99.85% in purity were precipitated from stripped V (V) solution extracted from vanadium-bearing shale with a high vanadium precipitation percentage of 99.25% via the method of hydrothermal hydrogen reduction under a facile condition of initial solution pH of 6, reaction temperature of 523 K, H2 partial pressure of 4 MPa and reaction time of 2 h.
- (3)
- The phase transformation mechanism of the vanadium precipitation process proposed in this paper can be described as follows. In the reduction of activated hydrogen, V (V) in solution is first reduced to V2O5− in forms of NaV2O5 with Na+, then NaV2O5 is reduced to V (IV) in forms of VO2(H2O)0.5 which is further reduced and hydrolyzed to the VOOH, at last the VOOH dehydrate with each other and transform to the orthorhombic V2O3. This process can be summarized as serial reductions with the phase transformation of HxVyOz(2z−x−5y)− → NaV2O5 → VO2(H2O)0.5 → VOOH → V2O3.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Zhao, W.; Zhao, H.; Wang, L.; Li, D.; Qi, T. A novel synergistic extraction method for recovering vanadium (V) from high-acidity chloride leaching liquor. Sep. Purif. Technol. 2016, 165, 166–172. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S. Preparation high purity V2O5 from a typical low-grade refractory stone coal using a pyro-hydrometallurgical process. Minerals 2016, 6, 69. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N. Mechanism of enhancing extraction of vanadium from stone coal by roasting with MgO. Minerals 2017, 7, 33. [Google Scholar] [CrossRef]
- Skyllaskazacos, M.; Cao, L.Y.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. Cheminform 2016, 47, 1521–1543. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N.; Shi, Q. Optimal location of vanadium in muscovite and its geometrical and electronic properties by DFT calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, S.; Liu, T.; Chen, T.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. Ind. Eng. Chem. 2017, 45, 241–247. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.; Zhang, Y.; Huang, J. Effect of CaF2/CaO composite additive on roasting of vanadium-bearing stone coal and acid leaching kinetics. Minerals 2017, 7, 43. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting. Minerals 2016, 6, 26. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, P.; Li, S. A new process of extracting vanadium from stone coal. Int. J. Miner. Metall. Mater. 2010, 17, 381–388. [Google Scholar] [CrossRef]
- Li, X.; Xie, B. Extraction of vanadium from high calcium vanadium slag using direct roasting and soda leaching. Int. J. Miner. Metall. Mater. 2012, 19, 595–601. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 2011, 84–1186. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, L.; Li, Q.; Wang, X.; Xiang, X. Leaching of vanadium from stone coal with sulfuric acid. Rare Metals 2009, 28, 1–4. [Google Scholar] [CrossRef]
- Chen, X.; Lan, X.; Zhang, Q.; Ma, H.; Zhou, J. Leaching vanadium by high concentration sulfuric acid from stone coal. Trans. Non-Ferrous Metall. Soc. China 2010, 20, s123–s126. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, C.; Fan, G.; Li, M.; Li, C.; Li, X. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Non-Ferrous Metall. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Shi, L.; Hu, J.; Qiao, P. Recovery of vanadium during ammonium molybdate production using ion exchange. Hydrometallurgy 2010, 104, 317–321. [Google Scholar] [CrossRef]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Zhang, H. Main factors affecting precipitation of vanadium with acidic ammonium salt and its countermeasures. Iron Alloy 2012, 4, 12–16. (In Chinese) [Google Scholar]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Mitochondria as a target for decavanadate toxicity in sparus aurata heart. Aquat. Toxicol. 2007, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M. Decavanadate toxicology and pharmacological activities: V10 or V1, both or nandone? Oxid. Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Ohlin, A.C.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Ohlin, A.C. Decavanadate in vitro and in vivo effects: Facts and opinions. J. Inorg. Biochem. 2014, 137, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Kumar, V.; Pandey, B.D.; Sahu, K.K. A comprehensive review on the hydro metallurgical process for the production of nickel and copper powders by hydrogen reduction. Mater. Res. Bull. 2006, 41, 879–892. [Google Scholar] [CrossRef]
- Yu, K.; Liang, H. Attempt at preparing Cu by direct hydrogen reduction of Cu2S slurry. Mater. Lett. 2004, 58, 3045–3048. [Google Scholar] [CrossRef]
- Saarinen, T.; Lindfors, L.E.; Fugleberg, S. A review of the precipitation of nickel from salt solutions by hydrogen reduction. Hydrometallurgy 1998, 47, 309–324. [Google Scholar] [CrossRef]
- Liang, H.; Tang, Q.; Yu, K.; Li, S.; Ke, J. Preparation of metallic silver from Ag2S slurry by direct hydrogen reduction under hydrothermal conditions. Mater. Lett. 2007, 61, 1020–1022. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, C.; Li, M.; Qiu, S.; Li, X. Thermodynamics of vanadium-sulfur-water systems at 298 K. Hydrometallurgy 2011, 106, 104–112. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S.; Shen, C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. [Google Scholar] [CrossRef]












| Composition | V2O5 | Fe2O3 | Al2O3 | MgO | Na2O | K2O | CaO | SiO2 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.71 | 4.99 | 8.91 | 2.18 | 0.38 | 3.02 | 6.26 | 49.28 | 4.35 | 17.82 |
| Minerals | V2O5 | SiO2 | Al2O3 | MgO | K2O | CaO | FeO |
|---|---|---|---|---|---|---|---|
| Muscovite | 3.52 | 49.09 | 27.31 | 4.49 | 9.56 | 0.02 | 0.18 |
| Quartz | 0 | 98.43 | 0 | 0 | 0 | 0 | 0 |
| Pyrite | 0 | 0.05 | 0 | 0 | 0 | 0 | 59.13 |
| Calcite | 0 | 0 | 0 | 1.10 | 0 | 65.94 | 0 |
| Contents | Vanadium Valence State | ||
|---|---|---|---|
| V (III) | V (IV) | V (V) | |
| Raw ore | 71 | 29 | 0 |
| Blank roasted ore | 44 | 52 | 4 |
| Leaching solution | 0 | 0 | 100 |
| Items | V | Fe | Al | Mg | Na | K | Ca | P | Si |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 20.32 | 0.009 | 0.014 | 0.008 | 23.05 | 0 | 0 | 0.021 | 0.013 |
| Composition | V2O3 | Fe | Si | P | S | K2O | Na2O | As |
|---|---|---|---|---|---|---|---|---|
| Content | 99.92 | 0.001 | 0.003 | 0.008 | 0.002 | 0 | 0.03 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, Y.; Bao, S.; Huang, J.; Zhang, L. A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology. Minerals 2017, 7, 182. https://doi.org/10.3390/min7100182
Zhang G, Zhang Y, Bao S, Huang J, Zhang L. A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology. Minerals. 2017; 7(10):182. https://doi.org/10.3390/min7100182
Chicago/Turabian StyleZhang, Guobin, Yimin Zhang, Shenxu Bao, Jing Huang, and Liuhong Zhang. 2017. "A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology" Minerals 7, no. 10: 182. https://doi.org/10.3390/min7100182
APA StyleZhang, G., Zhang, Y., Bao, S., Huang, J., & Zhang, L. (2017). A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology. Minerals, 7(10), 182. https://doi.org/10.3390/min7100182