New Mineral with Modular Structure Derived from Hatrurite from the Pyrometamorphic Rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel
Abstract
:1. Introduction
2. Methods of Investigation
3. Results
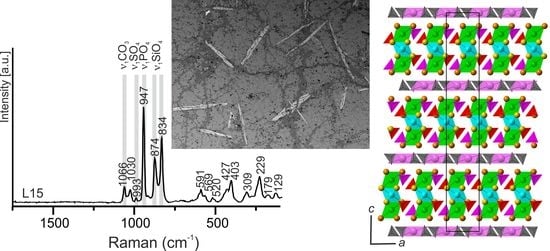
3.1. Occurrence and Description of Holotype Specimen
3.2. Occurrences of Ariegilatite in Other Localities of the Hatrurim Complex Rocks
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galuskin, E.V.; Gfeller, F.; Armbruster, T.; Galuskina, I.O.; Vapnik, Ye.; Murashko, M.; Wodyka, R.; Dzierżanowski, P. New minerals with modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex, Part I: Nabimusaite, KCa12(SiO4)4(SO4)2O2F, from larnite rock of the Jabel Harmun, Palestinian Autonomy, Israel. Mineral. Mag. 2015, 79, 1061–1072. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Gfeller, F.; Galuskin, E.V.; Armbruster, T.; Vapnik, Ye.; Dulski, M.; Gardocki, M.; Jeżak, L.; Murashko, M. New minerals with modular structure derived from hatrurite 1 from the pyrometamorphic rocks, part IV: Dargaite, CaCa12(SiO4)4(SO4)2O3, from Nahal Darga, Palestinian Autonomy. Mineral. Mag. 2018, 82. in press. [Google Scholar]
- Bentor, Y.K. (Ed.) Israel. In Lexique Stratigraphique International, Asie; CNRS: Paris, France, 1960; Volume III, Chapter 10.2. [Google Scholar]
- Gross, S. The Mineralogy of the Hatrurim Formation, Israel; Geological Survey of Israel: Jerusalem, Israel, 1977.
- Vapnik, Y.; Sharygin, V.V.; Sokol, E.V.; Shagam, R. Paralavas in a combustion metamorphic complex: Hatrurim Basin, Israel. Rev. Eng. Geol. 2007, 18, 1–21. [Google Scholar]
- Sokolova, E.V.; Yamnova, N.A.; Egorov-Tismenko, Y.K.; Khomyakov, A.P. The crystal structure of a new sodium-calcium-barium phosphate of Na, Ca and Ba (Na5Ca)Ca6Ba(PO4)6F3. Dokl. Akad. Nauk SSSR 1984, 274, 78–83. [Google Scholar]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Armbruster, T.; Krzątała, A.; Vapnik, Ye.; Kusz, J.; Dulski, M.; Gardocki, M.; Gurbanov, A.G.; et al. New minerals with a modular structure derived from hatrurite from the pyrometamorphic rocks. Part III. Gazeevite, BaCa6(SiO4)2(SO4)2O, from Israel and the Palestine Autonomy, South Levant, and from South Ossetia, Greater Caucasus. Mineral. Mag. 2017, 81, 499–513. [Google Scholar] [CrossRef]
- Sokolova, E.; Hawthorne, F.C. The crystal chemistry of the [M3φ11–14] trimeric structures: From hyperagpaitic complexes to saline lakes. Can. Mineral. 2001, 39, 1275–1294. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Pakhomova, A.; Armbruster, T.; Vapnik, Y.; Włodyka, R.; Dzierżanowski, P.; Murashko, M. New minerals with modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex, Part II: Zadovite, BaCa6[(SiO4)(PO4)](PO4)2F, and aradite, BaCa6[(SiO4)(VO4)](VO4)2F, from paralavas of the Hatrurim Basin, Negev Desert, Israel. Mineral. Mag. 2015, 79, 1073–1087. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Krüger, B.; Galuskina, I.O.; Krüger, H.; Vapnik, Ye.; Pauluhn, A.; Olieric, V. Stracherite, BaCa6(SiO4)2[(PO4)(CO3)]F, a first CO3-bearing intercalated hexagonal antiperovskite from Negev Desert, Israel. Amer. Mineral. 2018. under review. [Google Scholar]
- Waltersperger, S.; Olieric, V.; Pradervand, C.; Glettig, W.; Salathe, M.; Fuchs, M.R.; Curtin, A.; Wang, X.; Ebner, S.; Panepucci, E.; et al. PRIGo: A new multi-axis goniometer for macromolecular crystallography. J. Synchrotron Radiat. 2015, 22, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Wojdyla, J.A.; Kaminski, J.W.; Panepucci, E.; Ebner, S.; Wang, X.; Gabadinho, J.; Wang, M. DA+ data acquisition and analysis software at the Swiss Light Source macromolecular crystallography beamlines. J. Synchrotron Radiat. 2018, 25, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. D 2010, 166, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Krüger, B.; Galuskin, E.V.; Galuskina, I.O.; Krüger, H.; Vapnik, Y.; Olieric, V.; Pauluhn, A. A potentially new mineral with a modular structure based on antiperovskite layers. Mitt. Österr. Miner. Ges. 2017, 163, 59. [Google Scholar]
- Comodi, P.; Liu, Y. CO3 substitution in apatite: Further insight from new crystal-chemical data of Kasekere (Uganda) apatite. Eur. J. Mineral. 2000, 12, 965–974. [Google Scholar] [CrossRef]
- Banno, Y.; Miyawaki, R.; Momma, K.; Bunno, M. CO3-bearing member of the hydroxylapatite-hydroxylellestadite series from Tadano, Fukushima Prefecture, Japan: CO3-SO4 substitution in the apatite–ellestadite series. Mineral. Mag. 2016, 80, 363–370. [Google Scholar] [CrossRef]
- Jeffery, J.W. The crystal structure of tricalcium silicate. Acta Crystallogr. 1952, 5, 26–35. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Minerals with antiperovskite structure: A review. Z. Kristallogr. 2008, 223, 109–113. [Google Scholar] [CrossRef]
- Geller, Y.I.; Burg, A.; Halicz, L.; Kolodny, Y. System closure during the combustion metamorphic “Mottled Zone” event, Israel. Chem. Geol. 2012, 334, 25–36. [Google Scholar] [CrossRef]
- Novikov, I.; Vapnik, E.; Safonova, I. Mud volcano origin of the Mottled Zone, South Levant. Geosci. Front. 2013, 4, 597–619. [Google Scholar] [CrossRef]
- Khoury, H.N.; Salameh, E.M.; Clark, I.D. Mineralogy and origin of surficial uranium deposits hosted in travertine and calcrete from central Jordan. Appl. Geochem. 2014, 43, 49–65. [Google Scholar] [CrossRef]
- Sokol, E.V.; Seryotkin, Y.V.; Kokh, S.N.; Vapnik, Y.; Nigmatulina, E.N.; Goryainov, S.V.; Belogub, E.V.; Sharygin, V.V. Flamite, (Ca,Na,K)2(Si,P)O4, a new mineral from ultra high temperature combustion metamorphic rocks, Hatrurim Basin, Negev Desert, Israel. Mineral. Mag. 2015, 79, 583–596. [Google Scholar] [CrossRef]
- Gfeller, F.; Widmer, R.; Krüger, B.; Galuskin, E.V.; Galuskina, I.O.; Armbruster, T. The crystal structure of flamite and its relation to Ca2SiO4 polymorphs and nagelschmidtite. Eur. J. Mineral. 2015, 27, 755–769. [Google Scholar] [CrossRef]
- Fayos, J.; Glasser, F.P.; Howie, R.A.; Lachowski, E.; Perez-Mendez, M. Structure of dodecacalcium potassium fluoride dioxide terasilicate bis (sulphate), KF.2[Ca6(SO4)(SiO4)2O]: A fluorine containing phase encountered in cement clinker production process. Acta Crystallogr. C 1985, C41, 814–816. [Google Scholar] [CrossRef]
- Krüger, H. Ca5.45Li3.55[SiO4]3O0.45F1.55 and Ca7K[SiO4]3F3: Single-crystal synthesis and structures of two trigonal oxyfluorides. Z. Kristallogr. 2010, 225, 418–424. [Google Scholar] [CrossRef]
- Sokolova, E.; Hawthorne, F.C.; Khomyakov, A.P. Polyphite and sobolevite: Revision of their crystal structures. Can. Mineral. 2005, 43, 1527–1544. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Gfeller, F.; Krüger, B.; Kusz, J.; Vapnik, Ye.; Dulski, M.; Dzierżanowski, P. Silicocarnotite, Ca5[(SiO4)(PO4)](PO4), a new “old’’ mineral from the Negev Desert, Israel, and the ternesite–silicocarnotite solid solution: Indicators of high-temperature alteration of pyrometamorphic rocks of the Hatrurim Complex, Southern Levant. Eur. J. Mineral. 2016, 28, 105–123. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Prusik, K.; Vapnik, Ye.; Juroszek, R.; Jeżak, L.; Murashko, M. Dzierżanowskite, CaCu2S2—A new natural thiocuprate from Jabel Harmun, Judean Desert, Palestine Autonomy, Israel. Mineral. Mag. 2017, 81, 1073–1085. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]






| L15 | YV595 | SS20b | ||||||||
| Mean | 22 | s.d. | Range | 23 | s.d. | Range | 14 | s.d. | Range | |
| SO3 | 0.17 | 0.07 | 0.05–0.31 | 0.60 | 0.22 | 0.32–1.02 | n.d. | |||
| V2O5 | 0.10 | 0.06 | 0–0.17 | 0.32 | 0.08 | 0.13–0.51 | 0.41 | 0.07 | 0.26–0.52 | |
| P2O5 | 9.83 | 0.45 | 8.96–10.55 | 10.39 | 0.55 | 9.63–12.53 | 10.61 | 0.4 | 10.05–11.40 | |
| TiO2 | 0.12 | 0.06 | 0.05–0.25 | 0.31 | 0.04 | 0.24–0.40 | 0.26 | 0.16 | 0.10–0.56 | |
| SiO2 | 19.87 | 0.26 | 19.52–20.42 | 19.21 | 0.36 | 17.70–19.62 | 18.64 | 0.27 | 18.21–19.15 | |
| Fe2O3 | 0.34 | 0.09 | 0.17 | 0.05 | 0.11–0.24 | |||||
| Al2O3 | 0.12 | 0.03 | 0.07–0.18 | 0.17 | 0.11 | 0.10–0.68 | 0.24 | 0.06 | 0.17–0.38 | |
| BaO | 12.26 | 0.06 | 12.14–12.41 | 12.01 | 0.35 | 10.81–12.49 | 12.29 | 0.34 | 11.74–12.94 | |
| FeO | 0.32 | 0.06 | 0.24–0.46 | 0.49 | ||||||
| MnO | 0.29 | 0.08 | 0.09–0.39 | 0.26 | 0.07 | 0.14–0.41 | n.d. | |||
| CaO | 53.84 | 0.24 | 53.19–54.40 | 53.77 | 0.30 | 53.13–54.27 | 54.24 | 0.31 | 54.30–55.62 | |
| MgO | 0.14 | 0.03 | 0.11–0.22 | 0.38 | 0.05 | 0.27–0.53 | 0.03 | 0.02 | 0–0.07 | |
| K2O | 0.04 | 0.03 | 0–0.10 | n.d. | n.d. | |||||
| Na2O | 0.22 | 0.05 | 0.16–0.36 | 0.05 | 0.02 | 0.02–0.09 | 0.31 | 0.05 | 0.20–0.38 | |
| F | 3.17 | 0.10 | 2.96–3.34 | 3.24 | 0.45 | 2.27–3.27 | 3.05 | 0.11 | 2.84–3.17 | |
| CO2 * | 0.57 | 0.00 | 0.62 | |||||||
| –O=F | 1.33 | 1.36 | 1.28 | |||||||
| Total | 99.72 | 100.18 | 99.58 | |||||||
| Ba | 0.98 | 0.96 | 0.99 | |||||||
| K | 0.01 | |||||||||
| Na | 0.01 | 0.02 | 0.01 | |||||||
| Ca | 0.02 | |||||||||
| A | 1 | 1 | 1 | |||||||
| Ca | 11.77 | 11.75 | 11.88 | |||||||
| Mn2+ | 0.05 | 0.05 | ||||||||
| Fe2+ | 0.06 | 0.08 | ||||||||
| Mg | 0.04 | 0.12 | 0.01 | |||||||
| Na | 0.08 | 0.11 | ||||||||
| B | 12 | 12 | 12 | |||||||
| Si | 3.95 | 3.86 | 3.81 | |||||||
| Ti4+ | 0.02 | 0.05 | 0.04 | |||||||
| Fe3+ | 0.05 | 0.03 | ||||||||
| Al | 0.03 | 0.04 | 0.06 | |||||||
| P | 0.07 | |||||||||
| T1 | 4 | 4 | 4 | |||||||
| P | 1.70 | 1.80 | 1.77 | |||||||
| Si | 0.10 | 0.07 | ||||||||
| V5+ | 0.01 | 0.04 | 0.06 | |||||||
| S6+ | 0.03 | 0.09 | ||||||||
| C | 0.16 | 0.17 | ||||||||
| T2 | 2 | 2 | 2 | |||||||
| O | 0.96 | 0.91 | 1.03 | |||||||
| F | 2.04 | 2.09 | 1.97 | |||||||
| W | 2 | 2 | 2 | |||||||
| SS27A | MA5b | IS129 | teor | |||||||
| Mean | 29 | s.d. | Range | 11 | s.d. | Range | 12 | s.d. | Range | |
| SO3 | 0.05 | 0.08 | 0–0.34 | 1.35 | 0.22 | 1.10–1.92 | n.d. | |||
| V2O5 | 0.42 | 0.07 | 0.24–0.62 | 0.18 | 0.11 | 0.04–0.41 | n.d. | |||
| P2O5 | 10.52 | 0.52 | 9.48–11.65 | 12.91 | 0.43 | 11.89–13.60 | 9.02 | 0.49 | 8.31–10.16 | 11.53 |
| TiO2 | 0.27 | 0.16 | 0.05–0.59 | 0.18 | 0.06 | 0.09–0.28 | n.d. | |||
| SiO2 | 18.85 | 0.42 | 18.25–20.30 | 16.46 | 0.45 | 15.81–17.16 | 19.83 | 0.27 | 19.44–20.23 | 19.53 |
| Fe2O3 | n.d. | n.d. | ||||||||
| Al2O3 | 0.25 | 0.08 | 0.14–0.48 | 0.53 | 0.10 | 0.40–0.75 | 0.15 | 0.1 | 0.09–0.47 | |
| BaO | 12.26 | 0.41 | 11.39–13.15 | 12.17 | 0.49 | 11.42–13.31 | 12.36 | 0.17 | 12.12–12.66 | 12.46 |
| FeO | 0.17 | 0.11 | 0.03–0.38 | |||||||
| MnO | n.d. | n.d. | n.d. | |||||||
| CaO | 54.26 | 0.36 | 54.00–55.58 | 54.49 | 0.13 | 54.25–54.68 | 54.78 | 0.15 | 54.56–55.07 | 54.68 |
| MgO | 0.08 | 0.16 | 0–0.93 | 0.09 | 0.02 | 0.06–0.12 | n.d. | |||
| K2O | n.d. | 0.06 | 0.02 | 0.02–0.10 | 0.04 | 0.02 | 0.02–0.08 | |||
| Na2O | 0.24 | 0.08 | 0.06–0.37 | 0.23 | 0.04 | 0.15–0.32 | 0.66 | 0.11 | 0.49–0.82 | |
| F | 3.02 | 0.15 | 2.68–3.37 | 2.30 | 0.07 | 2.15–2.48 | 2.92 | 0.14 | 2.56–3.22 | 3.08 |
| CO2 * | 0.55 | 0.18 | 1.68 | 0.00 | ||||||
| –O=F | 1.27 | 0.97 | 1.23 | 1.29 | ||||||
| Total | 99.50 | 100.32 | 100.21 | 100.00 | ||||||
| Ba | 0.98 | 0.97 | 0.97 | 1.000 | ||||||
| K | 0.02 | 0.01 | ||||||||
| Na | 0.02 | 0.01 | 0.02 | |||||||
| Ca | ||||||||||
| A | 1 | 1 | 1 | |||||||
| Ca | 11.90 | 11.87 | 11.76 | 12.000 | ||||||
| Mn2+ | ||||||||||
| Fe2+ | 0.03 | |||||||||
| Mg | 0.02 | 0.03 | ||||||||
| Na | 0.08 | 0.07 | 0.24 | |||||||
| B | 12 | 12 | 12 | |||||||
| Si | 3.86 | 3.35 | 3.97 | 4.000 | ||||||
| Ti4+ | 0.04 | 0.02 | ||||||||
| Fe3+ | ||||||||||
| Al | 0.06 | 0.13 | 0.03 | |||||||
| P | 0.04 | 0.50 | ||||||||
| T1 | 4 | 4 | 4 | |||||||
| P | 1.78 | 1.72 | 1.53 | 2.000 | ||||||
| Si | 0.01 | |||||||||
| V5+ | 0.06 | 0.02 | ||||||||
| S6+ | 0.01 | 0.21 | ||||||||
| C | 0.15 | 0.05 | 0.46 | |||||||
| T2 | 2 | 2 | 2 | |||||||
| O | 1.04 | 1.52 | 1.15 | 1.000 | ||||||
| F | 1.96 | 1.48 | 1.85 | 2.000 | ||||||
| W | 2 | 2 | 2 | 2 | ||||||
| Crystal Data | |
| Crystal system | trigonal |
| Unit cell dimensions (Å) | a = 7.1551(6), c = 41.303(3) Å, b = 7.1551(6) c = 41.303(3) α, β = 90° γ = 120° |
| Space group | Rm (no.166) |
| Volume (Å3) | 1831.2(3) |
| Z | 3 |
| Density (calculated) | 3.329 g·cm−3 |
| Chemical formula | ~BaCa12(SiO4)4(PO4)1.8F2O |
| Crystal size (mm) | 38 × 32 × 25 μm |
| Data Collection | |
| Diffractometer | beamline X06DA, SLS multi-axis goniometer PRIGo PILATUS 2M-F detector λ = 0.70848 Å |
| Max. θ°-range for data collection | 32.139 |
| Index ranges | −10 ≤ h ≤ 6 |
| −8 ≤ k ≤ 10 | |
| −59 ≤ l ≤ 44 | |
| No. of measured reflections | 3445 |
| No. of unique reflections | 825 |
| No. of observed reflections (I > 2σ (I)) | 822 |
| Refinement of the Structure | |
| No. of parameters used in refinement | 58 |
| Rint | 0.0199 |
| Rσ | 0.0105 |
| R1, I > 2σ(I) | 0.0195 |
| R1 all Data | 0.0195 |
| wR2 on (F2) | 0.0555 |
| GooF | 1.128 |
| Δρ min (−e. Å−3) | −0.54 |
| Δρ max (e. Å−3) | 0.82 |
| Atom | x/a | y/b | z/c | sof | Ueq |
|---|---|---|---|---|---|
| Ba1 | 0 | 0 | 0 | 1 | 0.01731(9) |
| Ca1 | 0.16368(3) | 0.83632(3) | 0.39407(2) | 1 | 0.01059(12) |
| Ca2 | 0.15534(3) | 0.84466(3) | 0.53179(2) | 1 | 0.01036(12) |
| P1 | 0 | 0 | 0.67473(2) | 0.892(4) | 0.0085(2) |
| Si1 | 0 | 0 | 0.20615(2) | 1 | 0.00650(17) |
| Si2 | 0 | 0 | 0.08404(2) | 1 | 0.00723(17) |
| O1 | 0.55031(11) | 0.44969(11) | 0.64495(3) | 1 | 0.0148(4) |
| O2 | 0.12532(10) | 0.87468(10) | 0.19359(3) | 1 | 0.0130(4) |
| O3 | 0.12681(11) | 0.87319(11) | 0.07376(3) | 1 | 0.0140(4) |
| O4 | 0 | 0 | 0.36244(5) | 1 | 0.0125(5) |
| O5 | 0 | 0 | 0.75407(5) | 1 | 0.0132(5) |
| O6 | 0 | 0 | 0.12420(5) | 1 | 0.0113(5) |
| O7 | 0 | 0 | 0.5 | 1 | 0.0057(6) |
| F1 | 0 | 0 | 0.43179(4) | 1 | 0.0134(4) |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| Ba1 | 0.01604(12) | 0.01604(12) | 0.01985(15) | 0.00802(6) | 0 | 0 |
| Ca1 | 0.00850(14) | 0.00850(14) | 0.01499(17) | 0.00441(12) | 0.00013(5) | −0.00013(5) |
| Ca2 | 0.00855(14) | 0.00855(14) | 0.01337(17) | 0.00382(12) | −0.00066(5) | 0.00066(5) |
| P1 | 0.0087(3) | 0.0087(3) | 0.0082(4) | 0.00437(15) | 0 | 0 |
| Si1 | 0.0053(2) | 0.0053(2) | 0.0089(3) | 0.00264(11) | 0 | 0 |
| Si2 | 0.0063(2) | 0.0063(2) | 0.0091(3) | 0.00316(11) | 0 | 0 |
| O1 | 0.0156(4) | 0.0156(4) | 0.0144(5) | 0.0088(5) | 0.0010(2) | −0.0010(2) |
| O2 | 0.0113(4) | 0.0113(4) | 0.0194(6) | 0.0079(5) | 0.0009(2) | −0.0009(2) |
| O3 | 0.0107(4) | 0.0107(4) | 0.0220(6) | 0.0065(5) | 0.0015(2) | −0.0015(2) |
| O4 | 0.0136(6) | 0.0136(6) | 0.0103(8) | 0.0068(3) | 0 | 0 |
| O5 | 0.0147(6) | 0.0147(6) | 0.0102(8) | 0.0074(3) | 0 | 0 |
| O6 | 0.0114(6) | 0.0114(6) | 0.0110(8) | 0.0057(3) | 0 | 0 |
| O7 | 0.0059(7) | 0.0059(7) | 0.0054(10) | 0.0030(4) | 0 | 0 |
| F1 | 0.0131(5) | 0.0131(5) | 0.0140(8) | 0.0066(3) | 0 | 0 |
| Atom | -Atom | Distance | Atom | -Atom | Distance | ||
|---|---|---|---|---|---|---|---|
| Ba1 | -O1 | 2.8346(10) | ×6 | P1 | -O4 | 1.535(2) | |
| mean | 2.8346 | -O1 | 1.5484(11) | ×3 | |||
| Ca1 | -O5 | 2.3735(11) | mean | 1.5451 | |||
| -O3 | 2.4006(9) | ×2 | bvs | 4.859(9) | |||
| -O4 | 2.4128(12) | Si1 | -O2 | 1.6374(10) | ×3 | ||
| -O1 | 2.4628(12) | ×2 | -O5 | 1.643(2) | |||
| F1 | 2.5577(12) | mean | 1.6388 | ||||
| mean | 2.4387 | bvs | 3.843(7) | ||||
| bvs | 1.921(2) | Si2 | -O3 | 1.6279(10) | ×3 | ||
| Ca2 | -O6 | 2.2495(5) | -O6 | 1.659(2) | |||
| -F1 | 2.4431(12) | mean | 1.6356 | ||||
| -O2 | 2.3301(8) | ×2 | bvs | 3.879(7) | |||
| -O7 | 2.3303(5) | O7 | -Ca2 | 2.3303(5) | ×6 | ||
| -O2 | 2.5114(15) | mean | 2.3303 | ||||
| -O3 | 2.6028(15) | bvs | 2.248(1) | ||||
| mean | 2.3996 | F1 | -Ca1 | 2.5577(12) | ×3 | ||
| bvs | 2.196(2) | -Ca2 | 2.4431(12) | ×3 | |||
| bvs | 1.024(1) | mean | 2.5004 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galuskin, E.V.; Krüger, B.; Galuskina, I.O.; Krüger, H.; Vapnik, Y.; Wojdyla, J.A.; Murashko, M. New Mineral with Modular Structure Derived from Hatrurite from the Pyrometamorphic Rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel. Minerals 2018, 8, 109. https://doi.org/10.3390/min8030109
Galuskin EV, Krüger B, Galuskina IO, Krüger H, Vapnik Y, Wojdyla JA, Murashko M. New Mineral with Modular Structure Derived from Hatrurite from the Pyrometamorphic Rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel. Minerals. 2018; 8(3):109. https://doi.org/10.3390/min8030109
Chicago/Turabian StyleGaluskin, Evgeny V., Biljana Krüger, Irina O. Galuskina, Hannes Krüger, Yevgeny Vapnik, Justyna A. Wojdyla, and Mikhail Murashko. 2018. "New Mineral with Modular Structure Derived from Hatrurite from the Pyrometamorphic Rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel" Minerals 8, no. 3: 109. https://doi.org/10.3390/min8030109
APA StyleGaluskin, E. V., Krüger, B., Galuskina, I. O., Krüger, H., Vapnik, Y., Wojdyla, J. A., & Murashko, M. (2018). New Mineral with Modular Structure Derived from Hatrurite from the Pyrometamorphic Rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel. Minerals, 8(3), 109. https://doi.org/10.3390/min8030109