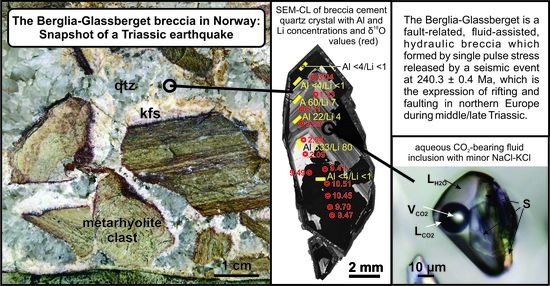
The Hydrothermal Breccia of Berglia-Glassberget, Trøndelag, Norway: Snapshot of a Triassic Earthquake
Abstract
:1. Introduction
2. Regional Geology and Characteristics of the Berglia-Glassberget Breccia
3. Sampling and Methods
3.1. Sampling
3.2. Scanning Electron Microscope Cathodoluminescence
3.3. Fluid Inclusion Microthermometry
3.4. Laser Ablation Inductively Coupled Plasma Mass Spectrometry
3.5. 40Ar/39Ar Dating of K-Feldspar
3.6. Secondary Ion Mass Spectrometry
4. Results
4.1. Chemistry of the Breccia Host Rock
4.2. Chemistry and Age of the K-Feldspar Cement
4.3. SEM-CL and Chemistry of the Quartz Cement
4.4. Petrography and Microthermometry of Fluid Inclusions
4.5. Oxygen Isotope Geochemistry of Quartz
5. Discussion
5.1. Processes Responsible for the Formation of the Berglia-Glassberget Breccia
5.2. Characteristics and Origin of Breccia-Forming Fluids
5.3. The Formation of the Berglia-Glassberget Breccia in the Regional Context
6. Summary
- The Berglia-Glassberget mineralization is a fluid-assisted, hydraulic breccia (250 m × 500 m in lateral dimension) which formed by single-pulse stress released by a seismic event during middle Triassic (240.3 ± 0.4 Ma).
- The influx of aqueous CO2-bearing Na-HCO3-SO4 fluids into the fault zone triggered a short-lived weakening mechanism that facilitated fault movement by reducing the shear stress.
- The intruding fluid, pumped by the seismic event to higher levels, leached K, Ba, Rb, Th, Nb and Ta metarhyolitic host rock and simultaneously silicified the host rock and its breccia fragments in the SW part of the shattered area (breccia “center”). The dissolved K, Ba, Rb and Th were precipitated mainly as breccia-cementing, low-temperature K-feldspar (var. adularia) followed by quartz.
- A high percentage of open space in the breccia fractures up to 80 vol % with cavities up 3 m × 3 m × 4 m in size, fluid inclusion microthermometry, and trace element chemistry of quartz suggest that the breccia was formed at depths between 4 and 0.5 km (1.1 to 0.1 kbar). The minimum temperature of the breccia cementing fluid was probably in the range of 247 and 329 °C. However, truly primary fluid inclusions could not be identified. The open space within the breccia body enabled the crystallization of large quantities of quartz crystals, which makes the locality attractive for mineral collectors.
- The origin of the CO2-bearing, breccia-cementing fluids may be of predominantly metamorphic origin due to decarbonation reactions (T > 200 °C) of limestones in an autochthonous sequence above felsic magmatic rocks of the underlying Olden Nappe. The decarbonation reactions were possibly initiated by deeply derived, hot fluids channelled to sub-surface levels by a major fault zone.
- In the regional context, the Berglia-Glassberget breccia is interpreted to be situated at a triple junction of long-lived fault zones belonging to the Møre-Trøndelag, Lærdal-Gjende and the Kollstraumen fault complexes. These fault systems are the expression of major rifting and faulting in northern Europe during middle/late Triassic. By this means the Berglia-Glassberget breccia contributes to a better understanding of the extensional tectonics of the Norwegian mainland during that period.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sibson, R.H. Fault rocks and fault mechanisms. J. Geol. Soc. 1977, 133, 191–213. [Google Scholar] [CrossRef]
- Sibson, R.H. Brecciation processes in fault zones: Inferences from earthquake rupturing. Pure Appl. Geophys. 1986, 124, 159–174. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Ore-related breccias in volcanoplutonic arcs. Econ. Geol. 1985, 80, 1467–1514. [Google Scholar] [CrossRef]
- Taylor, R.G.; Pollard, P.J. Mineralized Breccia Systems. Method of Recognition and Interpretation; Contributions of the Economic Geology Research Unit; Economic Geology Research Unit (EGRU): Townsville, Australia, 1993; Volume 46, p. 31. [Google Scholar]
- Fournier, R.O. Hydrothermal processes related to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ. Geol. 1999, 94, 1193–1211. [Google Scholar] [CrossRef]
- Landtwing, M.R.; Dillenbeck, E.D.; Leake, M.H.; Heinrich, C.A. Evolution of the breccia-hosted porphyry Cu-Mo-Au deposit at Agua Rica, Argentina: Progressive unroofing of a magmatic hydrothermal system. Econ. Geol. 2002, 97, 1273–1292. [Google Scholar] [CrossRef]
- Sibson, R.H. Earthquake faulting as a structural process. J. Struct. Geol. 1989, 11, 1–14. [Google Scholar] [CrossRef]
- Roberts, G.P. Displacement localization and palaeo-seismicity of the Rencurel Thrust Zone, French Sub-Alpine Chains. J. Struct. Geol. 1994, 16, 633–646. [Google Scholar] [CrossRef]
- Cowan, D.S. Do faults preserve a record of seismic slip? A field geologist’s opinion. J. Struct. Geol. 1999, 21, 995–1001. [Google Scholar] [CrossRef]
- Micklethwaite, S.; Cox, S.F. Fault-segment rupture, aftershock-zone fluid flow, and mineralization. Geology 2004, 32, 813–816. [Google Scholar] [CrossRef]
- Woodcock, N.H.; Dickson, J.A.D.; Tarasewicz, J.P.T. Transient fracture permeability and reseal hardening in fault zones: Evidence from dilation breccia textures. In Fractured Reservoirs; Lonergan, L., Jolly, R.J.H., Rawnsley, K., Sanderson, D.J., Eds.; Special Publications; Geological Society: London, UK, 2007; Volume 270, pp. 43–53. [Google Scholar]
- Baker, E.M.; Kirwin, D.J.; Taylor, R.G. Hydrothermal Breccia Pipes; Contributions of the Economic Geology Research Unit; Geology Department, James Cook University: Townsville, Australia, 1986; Volume 12, p. 45. [Google Scholar]
- Lawless, J.V.; White, P.J. Ore-related breccias: A revised genetic classification, with particular reference to epithermal deposits. In Proceedings of the 12th New Zealand Geothermal Workshop; Harvey, C.C., Browne, P.R.L., Freestone, D.H., Scott, G.L., Eds.; Geothermal Institute, University of Auckland: Auckland, New Zealand, 1990; pp. 197–202. [Google Scholar]
- Ewensson, T. Jakten på den Svarte Krystallen. Stein 2000, 27, 17–18. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Mineralogische Neuigkeiten aus Norwegen. Miner. Welt 2002, 13, 56–59. (In German) [Google Scholar]
- Jørgensen, L. Berglia-Glassberget kvartsforekomst, Sørli i Lierne. Nor. Bergverksmus. Skr. 2003, 25, 39–40. (In Norwegian) [Google Scholar]
- Asklund, B. Hauptzüge der Tektonik und Stratigraphie der Mittleren Kaledoniden in Schweden; C-417; Sveriges Geologiska Undersökning: Uppsala, Sweden, 1938; pp. 1–99. (In German) [Google Scholar]
- Johansson, L. Basement and Cover Relationships in the Vestranden-Grong-Olden Region, Central Scandinavian Caledonides: Petrology, Age Relationships, Structures and Regional Corrrelations. Ph.D. Thesis, Lund University, Lund, Sweden, 1986. [Google Scholar]
- Roberts, D. Tectonostratigraphy within the area of 1:50,000 map-sheet ‘Grong’, Nord-Trøndelag, Central Norway. Geol. Fören. Stockh. Förh. 1989, 111, 404–407. [Google Scholar] [CrossRef]
- Fossen, H.; Nissen, A.L.; Roberts, D. Bergrunnskart Blåfjellhatten 1923 III, M 1:50,000; Norges Geologiske Undersøkelse: Trondheim, Norway, 2013. [Google Scholar]
- Klingspor, I.; Tröeng, B. Rb-Sr and K-Ar age determinations of the Proterozoic Olden granite, Central Caledonides, Jämtland, Sweden. Geol. Fören. Stockh. Förh. 1980, 102, 515–522. [Google Scholar] [CrossRef]
- Roberts, D. Geologisk kart over Norge. Berggrunnsgeologisk kart Grong, M 1:250,000; Norges Geologiske Undersøkelse: Trondheim, Norway, 1997. [Google Scholar]
- Roberts, D. Geochemistry of Paleoproterozoic porphyritic felsic volcanites from the Olden and Tømmerås windows, Central Norway. Geol. Fören. Stockh. Förh. 1997, 119, 141–148. [Google Scholar]
- Roberts, D.; Nissen, A.L.; Walker, N. U-Pb zircon age and geochemistry of the Blåfjellhatten granite, Grong-Olden Culmination, Central Norway. Nor. Geol. Tidsskr. 1999, 79, 161–168. [Google Scholar] [CrossRef]
- Gorbatschev, R. Precambrian basement of the Caledonides. In The Caledonide Orogen—Scandinavia and Related Areas; Gee, D.G., Sturt, B.A., Eds.; John Wiley & Sons: Chichester, UK, 1985; pp. 197–212. [Google Scholar]
- Högdahl, K.; Andersson, U.B.; Eklund, O. The Transscandinavian Igneous Belt (TIB) in Sweden: A Review of Its Character and Evolution; Special Paper; Geological Survey of Finland: Espoo, 2004; Volume 37, p. 125. [Google Scholar]
- Lahtinen, R.; Garde, A.A.; Melezhik, V.A. Paleoproterozoic evolution of Fennoscandia and Greenland. Episodes 2008, 31, 20–28. [Google Scholar]
- Bingen, B.; Andersson, J.; Söderlun, U.; Möller, C. The Mesoproterozoic in the Nordic countries. Episodes 2008, 31, 29–34. [Google Scholar]
- Nordrum, F.S. Noen funn av mineraler i Norge 2000–2001, part II. Stein 2001, 28, 16–24. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2001–2002. Stein 2002, 29, 4–10. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2002–2003. Nor. Bergverksmus. Skr. 2003, 25, 82–89. [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2002–2003. Stein 2003, 30, 4–10. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2004–2005. Nor. Bergverksmus. Skr. 2005, 30, 117–124. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2006–2007. Stein 2007, 34, 14–26. (In Norwegian) [Google Scholar]
- Nordrum, F.S. Nyfunn av mineraler i Norge 2007–2008. Stein 2008, 35, 8–20. (In Norwegian) [Google Scholar]
- Jébrak, M. Hydrothermal breccias in vein-type ore deposits: A review of mechanisms, morphology and size distribution. Ore Geol. Rev. 1997, 12, 111–134. [Google Scholar] [CrossRef]
- ACMELabs, 2017. Bureau Veritas Mineral Laboratories. Available online: http://acmelab.com/ (accessed on 27 July 2017).
- Götze, J.; Plötze, M.; Habermann, D. Origin, spectral characteristics and practical applications of the cathodoluminescence (CL) of quartz—A review. Mineral. Petrol. 2001, 71, 225–250. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610-617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Müller, A.; Wiedenbeck, M.; Flem, B.; Schiellerup, H. Refinement of phosphorus determination in quartz by LA-ICP-MS through defining new reference material values. Geostand. Geoanal. Res. 2008, 32, 361–376. [Google Scholar] [CrossRef]
- McDougall, I.; Harrison, T.M. Geochronology and Thermochronology by the 40Ar/39Ar Method; Oxford University Press: New York, NY, USA, 1999; p. 269. [Google Scholar]
- Renne, P.R.; Mundil, R.; Balco, G.; Min, K.W.; Ludwig, K.R. Joint determination of K-40 decay constants and 40Ar/40K for the Fish Canyon sanidine standard, and improved accuracy for 40Ar/39Ar geochronology. Geochim. Cosmochim. Acta 2010, 74, 5349–5367. [Google Scholar] [CrossRef]
- Lee, J.Y.; Marti, K.; Severinghaus, J.P.; Kawamura, K.; Yoo, H.S.; Lee, J.B.; Kim, J.S. A redetermination of the isotopic abundances of atmospheric Ar. Geochim. Cosmochim. Acta 2006, 70, 4507–4512. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Reference Sheet for Reference Materials NBS 28 and NBS 30. 2007. Available online: https://nucleus.iaea.org/rpst/Documents/NBS28_NBS30.pdf (accessed on 20 February 2018).
- Baertschi, P. Absolute 18O content of standard mean ocean water. Earth Planet. Sci. Lett. 1976, 31, 341–344. [Google Scholar] [CrossRef]
- Ramsey, M.H.; Wiedenbeck, M. Quantifying isotopic heterogeneity of candidate Reference Materials at the picogram sampling scale. Geostand. Geoanal. Res. 2018, 42, 5–24. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivision of the A-type granitoids: Petrogenic and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Shand, S.J. Eruptive Rocks. Their Genesis, Composition, Classification, and Their Relation to Ore-Deposits with a Chapter on Meteorite; John Wiley & Sons: New York, NY, USA, 1943; p. 350. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 3, pp. 1–64. [Google Scholar]
- Teertstra, D.K.; Hawthorne, F.C.; Čerńy, P. Identification of normal and anomalous compositions of minerals by electron-microprobe analysis; K-rich feldspar as a case study. Can. Mineral. 1998, 36, 87–95. [Google Scholar]
- Correcher, V.; García-Guinea, J. On the luminescence properties of adularia feldspar. J. Lumin. 2001, 93, 303–312. [Google Scholar] [CrossRef]
- Sanchez-Munoz, L.; Müller, A.; Andrés, S.L.; Martin, R.F.; Modreski, P.J.; De Moura, O.J.M. The P-Fe diagram for K-feldspars: A preliminary approach in the discrimination of pegmatites. Lithos 2017, 272–273, 116–127. [Google Scholar] [CrossRef]
- Perny, B.; Eberhardt, P.; Ramseyer, K.; Mullis, J.; Pankrath, R. Microdistribution of Al, Li and Na in alpha quartz: Possible causes and correlation with short-lived cathodoluminescence. Am. Mineral. 1992, 77, 534–544. [Google Scholar]
- Rusk, B.G.; Reed, M.H.; Dilles, J.H.; Kent, A.J.R. Intensity of quartz cathodoluminescence and trace-element content in quartz from the porphyry copper deposit at Butte, Montana. Am. Mineral. 2006, 91, 1300–1312. [Google Scholar] [CrossRef]
- Rusk, B.G.; Lowers, H.; Reed, M.H. Trace elements in hydrothermal quartz; relationships to cathodoluminescent textures and insights into hydrothermal processes. Geology 2008, 36, 547–550. [Google Scholar] [CrossRef]
- Rusk, B. Cathodoluminescent textures and trace elements in hydrothermal quartz. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Heidelberg, Germany; New York, NY, USA, 2012; pp. 307–329. [Google Scholar]
- Jourdan, A.-L.; Vennemann, T.W.; Mullis, J.; Ramseyer, K.; Spiers, C.J. Evidence of growth and sector zoning in hydrothermal quartz from Alpine veins. Eur. J. Mineral. 2009, 21, 219–231. [Google Scholar] [CrossRef]
- Drivenes, K.; Larsen, B.R.; Müller, A.; Sørensen, B.E. Crystallization and uplift path of late-Variscan granites evidenced by quartz chemistry and fluid inclusions: An example from the Land’s End granite, SW England. Lithos 2016, 252–253, 57–75. [Google Scholar] [CrossRef]
- Garate-Olave, I.; Müller, A.; Roda-Robles, E.; Gil-Crespo, P.P.; Pesquera, A. Extreme fractionation in a granite-pegmatite system documented by quartz chemistry: The case study of Tres Arroyos (Central Iberian Zone, Spain). Lithos 2017, 286–287, 162–174. [Google Scholar] [CrossRef]
- Dennen, W.H. Stoichiometric substitution in natural quartz. Geochim. Cosmochim. Acta 1966, 30, 1235–1241. [Google Scholar] [CrossRef]
- Ramseyer, K.; Mullis, J. Factors influencing short-lived blue cathodoluminescence of a-quartz. Am. Mineral. 1990, 75, 791–800. [Google Scholar]
- Roedder, E. Fluid Inclusions; Reviews in Mineralogy; Mineralogical Society of America, Book Crafters, Inc.: Chelsea, MI, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Bakker, R.J. Package FLUIDS. Part 4: Thermodynamic modelling and purely empirical equations for H2O–NaCl–KCl solutions. Mineral. Petrol. 2012, 105, 1–29. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. HokieFlincs_H2O–NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O–NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Diamond, L.W. Introduction to gas-bearing aqueous fluid inclusions. Short Course Ser. Mineral. Assoc. Can. 2003, 32, 101–158. [Google Scholar]
- Onasch, C.M.; Vennemann, T.W. Disequilibrium partitioning of oxygen isotopes associated with sector zoning in quartz. Geology 1995, 23, 1103–1106. [Google Scholar] [CrossRef]
- Phillips, G.N.; Powell, R. Link between gold provinces. Econ. Geol. 1993, 88, 1084–1098. [Google Scholar] [CrossRef]
- Sibson, R.H. Earthquake rupturing as a mineralizing agent in hydrothermal systems. Geology 1987, 15, 701–704. [Google Scholar] [CrossRef]
- Pavlis, T.L.; Serpa, L.F.; Keener, C. Role of seismogenic processes in fault-rock development: An example from Death Valley, California. Geology 1993, 21, 267–270. [Google Scholar] [CrossRef]
- Williams, C.L.; Thompson, T.B.; Powell, J.L.; Dunbar, W.W. Goldbearing breccias of the Rain Mine, Carlin trend, Nevada. Econ. Geol. 2000, 95, 391–404. [Google Scholar] [CrossRef]
- Clark, C.; James, P. Hydrothermal brecciation due to fluid pressure fluctuations: Examples from the Olary Domain, South Australia. Tectonophysics 2003, 366, 187–206. [Google Scholar] [CrossRef]
- Labaume, P.; Carrio-Schaffhauser, E.; Gamond, J.-F.; Renard, F. Deformation mechanisms and fluid-driven mass transfers in the recent fault zones of the Corinth Rift (Greece). C. R. Geosci. 2004, 336, 375–383. [Google Scholar] [CrossRef]
- Collettini, C.; Cardellini, C.; Chiodini, G.; De Paola, N.; Holdsworth, R.E.; Smith, S.A.F. Fault weakening due to CO2 degassing in the Northern Apennines: Short- and long-term processes. Geol. Soc. Lond. Spec. Publ. 2008, 299, 175–194. [Google Scholar] [CrossRef]
- Sibson, R.H. Fluid flow accompanying faulting: Field evidence and models. In Earthquake Prediction: An International Review; Simpson, D.W., Richards, P.G., Eds.; Maurice Ewing Series 4; American Geophysical Union: Washington, DC, USA, 1981; pp. 593–603. [Google Scholar]
- Sibson, R.H. Implications of fault-valve behavior for rupture nucleation and recurrence. Tectonophysics 1992, 211, 283–293. [Google Scholar] [CrossRef]
- Tarasewicz, J.P.T.; Woodcock, N.H.; Dickson, J.A.D. Carbonate dilation breccias: Examples from the damage zone to the Dent Fault, northwest England. Geol. Soc. Am. Bull. 2005, 117, 736–745. [Google Scholar] [CrossRef]
- Grønlie, A.; Naeser, C.W.; Naeser, N.D.; Mitchell, J.G.; Sturt, B.A.; Ineson, P. Fission track and K/Ar dating of tectonic activity in a transect across the Møre Trøndelag Fault Zone, Central Norway. Nor. Geol. Tidsskr. 1994, 74, 24–34. [Google Scholar]
- Henley, R.W.; Ellis, A.J. Geothermal systems ancient and modern: A geochemical review. Earth Sci. Rev. 1983, 19, 1–50. [Google Scholar] [CrossRef]
- Henley, R.W. Epithermal gold deposits in volcanic terranes. In Gold Metallogeny and Exploration; Foster, R.P., Ed.; Springer: Boston, MA, USA, 1991; pp. 133–164. [Google Scholar]
- Huang, R.; Audétat, A. The titanium-in-quartz (TitaniQ) thermobarometer: A critical examination and re-calibration. Geochim. Cosmochim. Acta 2012, 84, 75–89. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1997. [Google Scholar]
- Klemd, R.; Hirdes, W. Origin of an unusual fluid composition in Early Proterozoic Palaeoplacer and lode-gold deposits in Birimian greenstone terranes of West Africa. S. Afr. J. Geol. 1997, 100, 405–414. [Google Scholar]
- Schmidt-Mumm, A.; Oberthür, T.; Vetter, U.; Blenkinsop, T.G. High CO2 content of fluid inclusions in gold mineralisations in the Ashanti Belt, Ghana: A new category of ore forming fluids? Miner. Depos. 1997, 32, 107–118. [Google Scholar] [CrossRef]
- Sibson, R.H. A brittle failure mode plot defining conditions for high-flux flow. Econ. Geol. 2000, 95, 41–48. [Google Scholar] [CrossRef]
- Lowenstern, B. Carbon dioxide in magmas and implications for hydrothermal systems. Miner. Depos. 2001, 36, 490–502. [Google Scholar] [CrossRef]
- Baker, T. Emplacement depth and carbon dioxide-rich fluid inclusions in intrusion-related gold deposits. Econ. Geol. 2002, 97, 1111–1117. [Google Scholar] [CrossRef]
- Kerrick, D.M.; Caldera, K. Metamorphic CO2 degassing from orogenic belts. Chem. Geol. 1998, 145, 213–232. [Google Scholar] [CrossRef]
- Santosh, M.; Jackson, D.H.; Harris, N.B.W.; Mattey, D.P. Carbonic fluid inclusions in South Indian granulites: Evidence for entrapment during charnockite formation. Contrib. Mineral. Petrol. 1991, 108, 318–330. [Google Scholar] [CrossRef]
- Wilmart, E.; Clocchiatti, R.; Duchesne, J.C.; Touret, J.L.R. Fluid inclusions in charnockites from the Bjerkreim–Sokndal massif (Rogaland, southwestern Norway): Fluid origin and in situ evolution. Contrib. Mineral. Petrol. 1991, 108, 453–462. [Google Scholar]
- Veevers, J.J. Middle/Late Triassic (230 ± 5 Ma) singularity in the stratigraphic and magmatic history of the Pangean heat anomaly. Geology 1989, 17, 784–787. [Google Scholar] [CrossRef]
- Ziegler, P.A. Evolution of sedimentary basins in North-West Europe. In Petroleum Geology of the Continental Shelf of North-West Europe; Institute of Petroleum: London, UK, 1981; pp. 3–39. [Google Scholar]
- Roberts, A.M.; Yielding, G.; Kusznir, N.J.; Walker, I.M.; Dorn-Lopez, D. Mesozoic extension in the North Sea: Constraints from flexural backstripping, forward modelling and fault populations. In Petroleum Geology of Northern Europe; Parker, J.R., Ed.; Geological Society: London, UK, 1993; pp. 1123–1136. [Google Scholar]
- Roberts, A.M.; Yielding, G.; Kusznir, N.J.; Walker, I.M.; Dorn-Lopez, D. Quantitative analysis of Triassic extension in the northern Viking Graben. J. Geol. Soc. 1995, 152, 15–26. [Google Scholar] [CrossRef]
- Færseth, R.B. Interaction of Permo-Triassic and Jurassic extensional fault-blocks during the development of the northern North Sea. J. Geol. Soc. 1996, 153, 931–944. [Google Scholar] [CrossRef]
- Eide, E.; Torsvik, T.H.; Andersen, T.B. Absolute dating of brittle fault movements: Late Permian and late Jurassic extensional fault breccias in western Norway. Terra Nova 1997, 9, 135–139. [Google Scholar] [CrossRef]
- Torsvik, T.H.; Andersen, T.B.; Eide, E.A.; Walderhaug, H.J. The age and tectonic significance of dolerite dykes in western Norway. J. Geol. Soc. 1997, 154, 961–973. [Google Scholar] [CrossRef]
- Frostick, L.; Reid, I.; Jarvis, J.; Eardley, H. Triassic sediments of the inner Moray Firth, Scotland: Early rift deposits. J. Geol. Soc. 1988, 145, 235–248. [Google Scholar] [CrossRef]
- Gabrielsen, R.H.; Ramberg, B. Fracture patterns in Norway from LandSAT imagery: results and potential use. In Proceedings of the Norwegian Sea Symposium Tromsø; NSS/23; Norwegian Petroleum Society: Stavanger, Norway, 1979; pp. 1–28. [Google Scholar]
- Doré, A.G.; Gage, M.S. Crustal alignments and sedimentary domains in the evolution of the North Sea, North East Atlantic Margin and Barents Shelf. In Petroleum Geology of North West Europe; Brooks, K., Glennie, K., Eds.; Graham and Trotman: London, UK, 1987; pp. 1131–1148. [Google Scholar]
- Grønlie, A.; Torsvik, T. On the origin and age of hydrothermal thorium enriched carbonate veins and breccias in the Møre Trøndelag Fault Zone, central Norway. Nor. Geol. Tidsskr. 1989, 69, 1–19. [Google Scholar]
- Grønlie, A.; Roberts, D. Resurgent strike-slip duplex development along the Hitra-Snåsa and Verran faults, Møre-Trøndelag Fault Zone, Central Norway. J. Struct. Geol. 1989, 11, 295–305. [Google Scholar] [CrossRef]
- Redfield, T.F.; Torsvik, T.H.; Andriessen, P.A.M.; Gabrielsen, R.H. Mesozoic and Cenozoic tectonics of the Møre Trøndelag Fault Complex, central Norway: Constraints from new apatite fission track data. Phys. Chem. Earth 2004, 29, 673–682. [Google Scholar] [CrossRef]
- Redfield, T.F.; Braathen, A.; Gabrielsen, R.H.; Osmundsen, P.T.; Torsvik, T.H.; Andriessen, P.A.M. Late Mesozoic to Early Cenozoic components of vertical separation across the Møre-Trøndelag Fault Complex, Norway. Tectonophysics 2005, 395, 233–249. [Google Scholar] [CrossRef]
- Gabrielsen, R.H.; Odinsen, T.; Grunnaleite, I. Structuring of the Northern Viking Graben and the Møre Basin; the influence of basement structural grain and the particular role of the Møre Trøndelag Fault Complex. Mar. Petrol. Geol. 1999, 16, 443–465. [Google Scholar] [CrossRef]
- Braathen, A. Kinematics of post-Caledonian polyphase brittle faulting in the Sunnfjord region, western Norway. Tectonophysics 1999, 302, 99–121. [Google Scholar] [CrossRef]
- Aanstad, K.; Gabrielsen, R.H.; Hagevang, T.; Ramberg, I.B.; Torvanger, O. Correlation of offshore and onshore structural features between 62N and 68N, Norway. In Proceedings of the Norwegian Symposium on Exploration; NSE/11; Norwegian Petroleum Society: Stavanger, Norway, 1981; pp. 1–25. [Google Scholar]
- Seranne, M. Late Paleozoic kinematics of the Møre Trøndelag Fault Zone and adjacent areas, central Norway. Nor. Geol. Tidskr. 1992, 72, 141–158. [Google Scholar]
- Andersen, T.B.; Torsvik, T.H.; Eide, E.; Osmundsen, P.T.; Faleide, J. Permian and Mesozoic extensional faulting within the Caledonides of Central South Norway. J. Geol. Soc. 1999, 156, 1073–1080. [Google Scholar] [CrossRef]
- Nordgulen, Ø.; Braathen, A.; Corfu, F.; Osmundsen, P.T.; Husmo, T. Polyphase kinematics and geochronology of the Kollstraumen detachment. Nor. J. Geol. 2002, 82, 299–316. [Google Scholar]
- Faleide, J.I.; Tsikalas, F.; Breivik, A.J.; Mjelde, R.; Ritzmann, O.; Engen, Ø.; Wilson, J.; Eldholm, O. Structure and evolution of the continental margin off Norway and the Barents Sea. Episodes 2008, 31, 82–91. [Google Scholar]
- Faleide, J.I.; Bjørlukke, K.; Gabrielsen, R.H.O. Geology of the Norwegian Continental Shelf. In Petroleum Geoscience: From Sedimentary Environments to Rock Physics; Bjørlukke, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Chapter 22; pp. 467–499. [Google Scholar]












| Sample nr. | 12071201 | 12071210 | 12071209 | 12071213 | 12071212 |
|---|---|---|---|---|---|
| Rock type | metarhyolite | metarhyolite | metarhyolite | silicified metarhyolite | silicified metarhyolite |
| Major elements (wt %) | |||||
| SiO2 | 70.78 | 72.75 | 75.63 | 93.1 | 97.29 |
| Al2O3 | 15.42 | 13.95 | 13.07 | 4.29 | 1.39 |
| Fe2O3 | 1.13 | 1.38 | 1.1 | 0.23 | 0.08 |
| MgO | 0.18 | 0.22 | 0.22 | 0.08 | 0.03 |
| CaO | 0.08 | 0.49 | 0.13 | 0.02 | 0.01 |
| Na2O | 3.27 | 3.84 | 3.22 | 0.03 | <0.01 |
| K2O | 7.92 | 6.13 | 5.83 | 1.29 | 0.28 |
| TiO2 | 0.28 | 0.34 | 0.26 | 0.18 | 0.16 |
| P2O5 | 0.04 | 0.04 | 0.02 | 0.03 | 0.02 |
| MnO | 0.01 | 0.04 | 0.02 | <0.01 | <0.01 |
| LOI | 0.8 | 0.7 | 0.4 | 0.7 | 0.7 |
| Sum | 99.88 | 99.88 | 99.93 | 99.97 | 99.98 |
| Trace elements (µg/g) | |||||
| Ba | 395 | 353 | 124 | 37 | 9 |
| Be | 1 | 3 | 3 | <1 | <1 |
| Cs | 2.9 | 1.8 | 3.1 | 0.7 | 0.1 |
| Ga | 12.4 | 14.7 | 14.4 | 4.8 | 1.7 |
| Hf | 7.9 | 8.6 | 7.2 | 3.4 | 3.8 |
| Mo | <0.1 | 0.2 | <0.1 | <0.1 | <0.1 |
| Pb | 5.2 | 13.3 | 3.3 | 2.9 | 1.1 |
| Nb | 21.1 | 21.1 | 22.6 | 4.9 | 3.9 |
| Rb | 333.4 | 226.9 | 244.7 | 70.6 | 18.3 |
| Sn | 2 | 3 | 2 | <1 | <1 |
| Sr | 48.6 | 61.7 | 44.4 | 28 | 30.1 |
| Ta | 1.3 | 1.3 | 1.5 | 0.4 | 0.3 |
| Th | 16.3 | 17.4 | 14.9 | 6.8 | 6.9 |
| U | 4 | 3.9 | 4 | 1.6 | 0.8 |
| W | 2.4 | 0.9 | 0.9 | 0.9 | 1.7 |
| Zr | 268.7 | 294.2 | 221.4 | 118 | 123.7 |
| Y | 28.6 | 30.6 | 18.4 | 10.2 | 10.2 |
| La | 15.7 | 32.3 | 7.8 | 11.3 | 17.3 |
| Ce | 57.4 | 76.3 | 68.3 | 35.9 | 34.1 |
| Pr | 3.23 | 7.27 | 1.79 | 1.97 | 2.94 |
| Nd | 11.1 | 25.5 | 6.3 | 6.2 | 9.7 |
| Sm | 2.03 | 4.35 | 1.36 | 1.04 | 1.43 |
| Eu | 0.2 | 0.48 | 0.09 | 0.16 | 0.18 |
| Gd | 2.47 | 3.9 | 1.57 | 1.01 | 1.25 |
| Tb | 0.51 | 0.65 | 0.36 | 0.23 | 0.24 |
| Dy | 4.18 | 4.49 | 2.56 | 1.72 | 1.56 |
| Ho | 0.92 | 1.12 | 0.68 | 0.38 | 0.33 |
| Er | 3.31 | 3.27 | 2.49 | 1.19 | 1.1 |
| Tm | 0.54 | 0.55 | 0.43 | 0.2 | 0.19 |
| Yb | 3.54 | 3.81 | 3.3 | 1.15 | 1.34 |
| Lu | 0.6 | 0.61 | 0.55 | 0.19 | 0.22 |
| Major Elements (wt %) | Trace Elements (µg/g) | ||||
|---|---|---|---|---|---|
| SiO2 | 62.34 | Ba | 1937 | Y | 34.7 |
| TiO2 | 0.69 | Cs | 0.9 | La | 94.1 |
| Al2O3 | 17.88 | Ga | 11 | Ce | 190.1 |
| Fe2O3 | 0.6 | Hf | 23 | Pr | 18.07 |
| MnO | <0.01 | Nb | 28.1 | Nd | 57.6 |
| MgO | 0.03 | Pb | 25.4 | Sm | 8.68 |
| CaO | 0.31 | Rb | 792 | Eu | 0.95 |
| Na2O | 0.08 | Sr | 70.2 | Gd | 6.24 |
| K2O | 16.11 | Ta | 2.0 | Tb | 0.86 |
| P2O5 | 0.24 | Th | 77 | Dy | 5.39 |
| LOI | 1.3 | U | 7.9 | Ho | 1.08 |
| Sum | 99.58 | V | 46 | Er | 4.01 |
| An | 1.6 | W | 22.1 | Tm | 0.68 |
| Ab | 0.7 | Zn | 13 | Yb | 5.59 |
| Or | 97.7 | Zr | 814.2 | Lu | 0.97 |
| UTM 33W | Spectrum | Inverse Isochron | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample nr. | Material | E | N | Steps(n) | %39Ar | Age ± 1.96σ | MSWD(P) | TGA ± 1.96σ | K/Ca ± 1.96σ | Age ± 1.96σ | MSWD (P) | Trapped 40Ar/36Ar | Spread (%) |
| 12071206 | K-Feldspar | 426849 | 7123189 | 19–25(7) | 51.26 | 240.26 ± 0.36 | 0.59(0.74) | 238.38 ± 0.39 | 72.82 ± 2.74 | 240.19 ± 3.87 | 0.76(0.58) | 298.71 ± 9.02 | 5.6 |
| CL Intensity | Li | Mn | Ge | Sr | Al | P | Ca | |
|---|---|---|---|---|---|---|---|---|
| LOD | 0.8 | 0.2 | 0.07 | 0.03 | 4.2 | 5.0 | 6.3 | |
| 12071204-A | moderate | 26.0 | 0.2 | 2.83 | 0.06 | 284.3 | <5.0 | <6.3 |
| 12071204-B | high | 217.9 | 0.2 | 0.08 | 0.12 | 1707.6 | <5.0 | <6.3 |
| 12071204-C | moderate | 115.3 | 0.6 | 0.07 | 0.14 | 260.6 | <5.0 | 11.5 |
| 12071204-D | high | 41.1 | 0.3 | <0.07 | <0.03 | 521.4 | 5.3 | <6.3 |
| 12071204-E | high | 12.5 | 0.3 | 0.08 | <0.03 | 742.2 | 10.0 | 13.9 |
| 12071204-F | high | 165.2 | 0.3 | 0.07 | <0.03 | 1408.9 | <5.0 | <6.3 |
| 12071206-A | high | 24.3 | <0.2 | <0.07 | 0.09 | 278.1 | 6.7 | <6.3 |
| 12071206-B | moderate | 14.1 | 0.4 | <0.07 | 0.09 | 55.7 | 7.2 | <6.3 |
| 12071206-C | low | 4.8 | 0.3 | <0.07 | 0.05 | 109.0 | 8.2 | <6.3 |
| 12071206-D | moderate | 18.1 | 0.2 | <0.07 | 0.04 | 262.6 | 5.4 | 7.0 |
| 12071206-E | high | 83.9 | 0.4 | 0.08 | <0.03 | 775.5 | 8.1 | 45.4 |
| 12071206-F | moderate | 28.7 | 0.4 | <0.07 | 0.14 | 196.3 | 7.0 | <6.3 |
| 12071215-A | low | 96.8 | <0.2 | 15.03 | 0.15 | 1634.3 | <5.0 | <6.3 |
| 12071215-B | high | 59.3 | 0.3 | 3.45 | 0.07 | 210.6 | <5.0 | <6.3 |
| 12071215-C | high | 79.1 | 0.3 | <0.07 | 0.07 | 643.3 | 5.2 | 13.7 |
| 12071215-D | high | 128.1 | 0.5 | <0.07 | 0.18 | 505.1 | <5.0 | <6.3 |
| 12071215-E | low | 76.5 | 0.3 | 2.29 | 0.10 | 112.1 | <5.0 | 22.6 |
| 12071215-F | low | 80.0 | 0.4 | 2.71 | 0.10 | 16.7 | <5.0 | <6.3 |
| 12071217-A | very low | <0.8 | 0.4 | 0.29 | 0.04 | <4.2 | <5.0 | 21.4 |
| 12071217-B | very low | <0.8 | 0.4 | <0.07 | 0.06 | <4.2 | <5.0 | <6.3 |
| 12071217-C | low | 6.8 | 0.4 | <0.07 | <0.03 | 59.8 | 5.9 | 29.8 |
| 12071217-D | low | 3.7 | 0.4 | <0.07 | 0.05 | 22.4 | <5.0 | <6.3 |
| 12071217-E | high | 80.0 | 0.3 | 0.09 | 0.03 | 633.2 | <5.0 | <6.3 |
| 12071217-F | very low | <0.8 | 0.3 | 0.08 | 0.03 | <4.2 | <5.0 | 11.4 |
| 12071218-A | very low | <0.8 | 0.6 | <0.07 | 0.04 | <4.2 | <5.0 | <6.3 |
| 12071218-B | low | 50.6 | 0.2 | 0.17 | 0.05 | 164.0 | <5.0 | <6.3 |
| 12071218-C | very high | 316.2 | 0.3 | 0.14 | 0.07 | 2104.8 | 7.5 | <6.3 |
| 12071218-D | very high | 319.3 | 0.4 | 0.15 | 0.15 | 2471.3 | <5.0 | <6.3 |
| 12071218-E | very high | 297.2 | 0.4 | 0.11 | 0.11 | 1549.7 | <5.0 | <6.3 |
| 12071218-F | low | 3.2 | 0.4 | <0.07 | 0.03 | <4.2 | <5.0 | <6.3 |
| Type I | Type II | Type III | |
|---|---|---|---|
| inclusion type | as groups, pseudosecondary | as groups, pseudosecondary | in trails, pseudosecondary |
| inclusion petrography | multi-phase, isometric | multi-phase, isometric | two- and multi-phase, isometric to longish |
| size (µm) | 35 to 60 | 15 to 25 | 25 to 55 |
| degree of fill | 0.60 to 0.75 | 0.80 to 0.95 | 0.70 to 0.95 |
| Te (°C) | −24 to −22 | −29 to −24 | −41 to −21 |
| Tmice (°C) | −0.4 to −0.6 | −4.6 to 2.2 | −8.9 to 19.2 |
| Th (°C) | 242 to 248 | 203 to 214 | 136 to 189 |
| number of analyzed inclusions | 4 | 4 | 15 |
| 12071215—Breccia Center | 12071217—Breccia Margin | 12071206—Breccia Margin | |||
|---|---|---|---|---|---|
| Analysis nr. | δ18O (‰ ± 0.1) | Analysis nr. | δ18O (‰ ± 0.1) | Analysis nr. | δ18O (‰ ± 0.1) |
| 01 | 11.7 | 01 | 3.0 | 01 | 6.4 |
| 02 | 12.2 | 02 | 1.3 | 02 | 6.8 |
| 03 | 11.3 | 03 | 2.1 | 03 | 1.0 |
| 04 | 11.4 | 04 | 2.9 | 04 | 2.6 |
| 05 | 9.2 | 05 | 2.9 | 05 | 0.9 |
| 06 | 9.3 | 06 | 2.1 | 06 | 1.6 |
| 07 | 9.8 | 07 | 9.4 | 07 | 1.0 |
| 08 | 9.9 | 08 | 9.5 | 08 | 1.1 |
| 09 | 11.9 | 09 | 10.5 | 09 | 5.2 |
| 10 | 10.9 | 10 | 10.4 | 10 | 5.4 |
| 11 | 11.8 | 11 | 9.7 | 11 | 5.5 |
| 12 | 10.7 | 12 | 9.5 | 12 | 5.5 |
| 13 | 6.2 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, A.; Ganerød, M.; Wiedenbeck, M.; Svendsen Spjelkavik, S.O.; Selbekk, R. The Hydrothermal Breccia of Berglia-Glassberget, Trøndelag, Norway: Snapshot of a Triassic Earthquake. Minerals 2018, 8, 175. https://doi.org/10.3390/min8050175
Müller A, Ganerød M, Wiedenbeck M, Svendsen Spjelkavik SO, Selbekk R. The Hydrothermal Breccia of Berglia-Glassberget, Trøndelag, Norway: Snapshot of a Triassic Earthquake. Minerals. 2018; 8(5):175. https://doi.org/10.3390/min8050175
Chicago/Turabian StyleMüller, Axel, Morgan Ganerød, Michael Wiedenbeck, Skule Olaus Svendsen Spjelkavik, and Rune Selbekk. 2018. "The Hydrothermal Breccia of Berglia-Glassberget, Trøndelag, Norway: Snapshot of a Triassic Earthquake" Minerals 8, no. 5: 175. https://doi.org/10.3390/min8050175
APA StyleMüller, A., Ganerød, M., Wiedenbeck, M., Svendsen Spjelkavik, S. O., & Selbekk, R. (2018). The Hydrothermal Breccia of Berglia-Glassberget, Trøndelag, Norway: Snapshot of a Triassic Earthquake. Minerals, 8(5), 175. https://doi.org/10.3390/min8050175