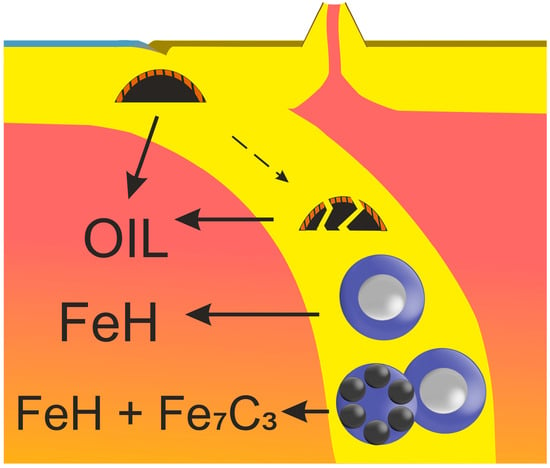
Fate of Hydrocarbons in Iron-Bearing Mineral Environments during Subduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Setup
2.3. Analytical Techniques
3. Results
3.1. Graphite And Hydrocarbons In Reaction Products
3.2. Formation of Iron Hydride, Iron Carbide and Graphite from Hydrocarbons and Iron-Bearing Materials
4. Discussion
4.1. Influence of Starting Iron-Bearing Material and Oxygen Fugacity on the Yield of Iron Hydride
4.2. Depth Intervals of Iron Hydride and Iron Carbide Formation, According to Slab Geotherms
4.3. α-Fe in the Reaction Products
4.4. Formation of Iron Hydride Phase in Mantle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sverjensky, D.A.; Stagno, V.; Huang, F. Important role for organic carbon in subduction-zone fluids in the deep carbon cycle. Nat. Geosci. 2014, 7, 909–913. [Google Scholar] [CrossRef]
- Facq, S.; Daniel, I.; Montagnac, G.; Cardon, H.; Sverjensky, D.A. In situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim. Cosmochim. Acta 2014, 132, 375–390. [Google Scholar] [CrossRef]
- Galvez, M.E.; Beyssac, O.; Martinez, I.; Benzerara, K.; Chaduteau, C.; Malvoisin, B.; Malavieille, J. Graphite formation by carbonate reduction during subduction. Nat. Geosci. 2013, 6, 473–477. [Google Scholar] [CrossRef]
- Dasgupta, R.; Mallik, A.; Tsuno, K.; Withers, A.C.; Hirth, G.; Hirschmann, M.M. Carbon-dioxide-rich silicate melt in the Earth’s upper mantle. Nature 2013, 493, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Kopf, A.J. Significance of mud volcanism. Rev. Geophys. 2002, 40, 2-1–2-52. [Google Scholar] [CrossRef]
- Evans, K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012, 113, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Zdrokov, E.V. Iron carbide as a source of carbon for graphite and diamond formation under lithospheric mantle P-T parameters. Lithos 2017, 286-287, 151–161. [Google Scholar] [CrossRef]
- Mann, P.; Gahagan, L.; Gordon, M.B. Tectonic Setting of the World’s Giant Oil and Gas Fields. In Giant Oil and Gas Fields of the Decade 1990-1999; AAPG Datapage: Tulsa, OK, USA, 2003; pp. 15–105. [Google Scholar]
- Karato, S.-I. Physics and Chemistry of the Deep Earth; Karato, S.-I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 416. [Google Scholar]
- Langdon, S.P.; Connor, J.K.; Chandler, R.B.; Jellison, M.J. Deepwater Drilling Challenges Demonstrate Learning Curve with New Connection Technology. In Proceedings of the 2010 IADC/SPE Drilling Conference and Exhibition, New Orleans, LO, USA, 2–4 February 2010. [Google Scholar] [CrossRef]
- Serovaiskii, A.Y. Investigation of stability of the model hydrocarbon system at thermobaric conditions, corresponding to depth down to 50 km. In Proceedings of the “Bakirovskie chteniya” conference, Moscow, Russia, 1 March 2018. [Google Scholar]
- Kolesnikov, A.; Kutcherov, V.G.; Goncharov, A.F. Methane-derived hydrocarbons produced under upper-mantle conditions. Nat. Geosci. 2009, 2, 566–570. [Google Scholar] [CrossRef]
- Kenney, J.F.; Kutcherov, V.A.; Bendeliani, N.A.; Alekseev, V.A. The evolution of multicomponent systems at high pressures: VI. The thermodynamic stability of the hydrogen-carbon system: The genesis of hydrocarbons and the origin of petroleum. Proc. Nalt. Acad. Sci. USA 2002, 99, 10976–10981. [Google Scholar] [CrossRef]
- Kutcherov, V.G.; Kolesnikov, A.; Dyugheva, T.I.; Kulikova, L.F.; Nikolaev, N.N.; Sazanova, O.A.; Braghkin, V.V. Synthesis of complex hydrocarbon systems at temperatures and pressures corresponding to the Earth’s upper mantle conditions. Dokl. Phys. Chem. 2010, 433, 132–135. [Google Scholar] [CrossRef]
- Frost, D.J.; McCammon, C.A. The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci. 2008, 36, 389–420. [Google Scholar] [CrossRef]
- Sinmyo, R.; Glazyrin, K.; McCammon, C.; Kupenko, I.; Kantor, A.; Potapkin, V.; Chumakov, A.I.; Rüffer, R.; Dubrovinsky, L. The influence of solid solution on elastic wave velocity determination in (Mg,Fe)O using nuclear inelastic scattering. Phys. Earth Planet. Inter. 2014, 229, 16–23. [Google Scholar] [CrossRef]
- Dewaele, A.; Datchi, F.; Loubeyre, P.; Mezouar, M. High pressure-high temperature equations of state of neon and diamond. Phys. Rev. B 2008, 77, 094106. [Google Scholar] [CrossRef]
- Kupenko, I.; Dubrovinsky, L.; Dubrovinskaia, N.; McCammon, C.; Glazyrin, K.; Bykova, E.; Ballaran, T.B.; Sinmyo, R.; Chumakov, A.I.; Potapkin, V.; et al. Portable double-sided laser-heating system for Mossbauer spectroscopy and X-ray diffraction experiments at synchrotron facilities with diamond anvil cells. Rev. Sci. Instrum. 2012, 83, 124501. [Google Scholar] [CrossRef]
- Kantor, I.; Prakapenka, V.; Kantor, A.; Dera, P.; Kurnosov, A.; Sinogeikin, S.; Dubrovinskaia, N.; Dubrovinsky, L. BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 2012, 83, 125102. [Google Scholar] [CrossRef]
- Potapkin, V.; Chumakov, A.I.; Smirnov, G.V.; Ruffer, R.; McCammon, C.; Dubrovinsky, L. Angular, spectral, and temporal properties of nuclear radiation from a Fe-57 synchrotron Mossbauer source. Phys. Rev. A 2012, 86, 053808. [Google Scholar] [CrossRef]
- Prescher, C.; McCammon, C.; Dubrovinsky, L. MossA: A program for analyzing energy-domain Mossbauer spectra from conventional and synchrotron sources. J. Appl. Crystallogr. 2012, 45, 329–331. [Google Scholar] [CrossRef]
- Hanfland, M.; Beister, H.; Syassen, K. Graphite under pressure: Equation of state and first-order Raman modes. Phys. Rev. B 1989, 39, 12598. [Google Scholar] [CrossRef]
- Sterin, K.E.; Aleksanyan, V.; Zhizhin, G.N. Raman Spectra of Hydrocarbons; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Narygina, O.; Dubrovinsky, L.S.; McCammon, C.A.; Kurnosov, A.; Kantor, I.Y.; Prakapenka, V.B.; Dubrovinskaia, N.A. X-ray diffraction and Mössbauer spectroscopy study of fcc iron hydride FeH at high pressures and implications for the composition of the Earth’s core. Earth Planet. Sci. Lett. 2011, 307, 409–414. [Google Scholar] [CrossRef]
- Prescher, C.; Dubrovinsky, L.; Bykova, E.; Kupenko, I.; Glazyrin, K.; Kantor, A.; McCammon, C.; Mookherjee, M.; Nakajima, Y.; Miyajima, N.; et al. High Poisson’s ratio of Earth’s inner core explained by carbon alloying. Nat. Geosci. 2015, 8, 220–223. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Sakamaki, K.; Takahashi, E.; Nakajima, Y.; Nishihara, Y.; Funakoshi, K.; Suzuki, T.; Fukai, Y. Melting phase relation of FeHx up to 20 GPa: Implication for the temperature of the Earth’s core. Phys. Earth Planet. Inter. 2009, 174, 192–201. [Google Scholar] [CrossRef]
- Badding, J.V.; Hemley, R.J.; Mao, H.K. High-pressure chemistry of hydrogen in metals—In situ study of iron hydride. Science 1991, 253, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Fukai, Y.; Yamakata, M.; Yagi, T. Some High-Pressure Experiments on the Fe-H System. Z. Phys. Chem. 1993, 179, 119–123. [Google Scholar] [CrossRef]
- Yamakata, M.; Yagi, T.; Utsumi, W.; Fukai, Y. In situ X-ray Observation of Iron Hydride under High Pressure and High Temperature. Proc. Jpn. Acad. Ser. B 1992, 68, 172–176. [Google Scholar] [CrossRef]
- Pépin, C.M.; Dewaele, A.; Geneste, G.; Loubeyre, P.; Mezouar, M. New Iron Hydrides under High Pressure. Phys. Rev. Lett. 2014, 113, 265504. [Google Scholar] [CrossRef]
- Shibazaki, Y.; Terasaki, H.; Ohtani, E.; Tateyama, R.; Nishida, K.; Funakoshi, K.-i.; Higo, Y. High-pressure and high-temperature phase diagram for Fe0.9Ni0.1–H alloy. Phys. Earth Planet. Inter. 2014, 228, 192–201. [Google Scholar] [CrossRef]
- Lord, O.T.; Walter, M.J.; Dasgupta, R.; Walker, D.; Clark, S.M. Melting in the Fe-C system to 70 GPa. Earth Planet. Sci. Lett. 2009, 284, 157–167. [Google Scholar] [CrossRef]
- Nakajima, Y.; Takahashi, E.; Suzuki, T.; Funakoshi, K. “Carbon in the core” revisited. Phys. Earth Planet. Inter. 2009, 174, 202–211. [Google Scholar] [CrossRef]
- Takahashi, S.; Ohtani, E.; Sakai, T.; Mashino, I.; Kamasda, S.; Miyahara, M.; Sakamaki, T.; Hirao, N.; Ohishi, Y. Stability and melting relations of Fe3C up to 3 Mbar: Implication for the carbon in the Earth’s inner core. In Proceedings of the American Geophysical Union, San Francisco, CA, USA, 9–13 December 2013. [Google Scholar]



| Exp # | System | Final P, (±0.2) GPa | T, (±100) K | Mössbauer Spectroscopy Results | Raman Results | X-ray Diffraction Results | |
|---|---|---|---|---|---|---|---|
| Rel. Area % | Assignment | ||||||
| 1 | Paraffin oil + pyroxene-like glass (Mg0.91Fe0.09)(Si0.91Al0.09)O3 | 2.6 | 1500 | 81.7 | pyroxene glass | Graphite | |
| 18.3 | FeH | ||||||
| 2 | Paraffin oil + pyroxene-like glass (Mg0.91Fe0.09)(Si0.91Al0.09)O3 | 4.5 | 1500 | 100.0 | pyroxene glass | - | - |
| 3 | 1800 | 66.4 | pyroxene glass | Graphite + hydrocarbons | orthorhombic Fe7C3 + fcc FeH + dhcp-FeH + graphite + clinopyroxene | ||
| 33.6 | FeH | ||||||
| 4 | Crude oil + ferropericlase (Mg0.8Fe0.2)O | 6.9 | 1300 | 100.0 | ferropericlase | Fluorescence typical for crude oil | |
| 5 | 1600 | 37.8 | ferropericlase | ||||
| 34.2 | new Fe2+ component | ||||||
| 28.0 | FeH | ||||||
| 6 | 1800 | 26.1 | new Fe2+ component | ||||
| 53.1 | FeH | ||||||
| 20.8 | α-Fe | ||||||
| 7 | Paraffin oil + Fe0.94O | 7.5 | 1200 | 100.0 | Fe0.94O | Graphite + hydrocarbons | |
| 8 | 1400 | 15.5 | Mixed phases | Graphite + hydrocarbons | orthorhombic Fe7C3 + dhcp-FeH + graphite + FeO | ||
| 7.9 | FeH | ||||||
| 34.2 | Fe7C3a | ||||||
| 38.0 | Fe7C3b | ||||||
| 4.4 | α-Fe | ||||||
| 9 | 1600 | 15.7 | Mixed phases | Graphite + hydrocarbons | orthorhombic Fe7C3 + dhcp-FeH + graphite + FeO | ||
| 11.7 | FeH | ||||||
| 19.2 | Fe7C3a | ||||||
| 44.6 | Fe7C3b | ||||||
| 8.9 | α-Fe | ||||||
| 10 | 2300 | 9.5 | Mixed phases | Graphite + hydrocarbons | orthorhombic Fe7C3 + dhcp-FeH + graphite | ||
| 37.9 | FeH | ||||||
| 23.3 | Fe7C3a | ||||||
| 19.9 | Fe7C3b | ||||||
| 9.4 | α-Fe | ||||||
| 11 | Paraffin oil + ferropericlase (Mg0.75Fe0.25)O | 7.4 | 1200 | 100.0 | ferropericlase | Graphite + hydrocarbons | |
| 12 | 1600 | 100.0 | ferropericlase | Graphite + hydrocarbons | |||
| 13 | 1800 | 53.4 | ferropericlase | Graphite + hydrocarbons | |||
| 19.2 | FeH | ||||||
| 19.3 | Fe7C3 | ||||||
| 8.1 | α-Fe | ||||||
| 14 | Paraffin oil + pyroxene-like glass (Mg0.91Fe0.09)(Si0.91Al0.09)O3 | 8.8 | 1600 | 84.6 | pyroxene glass | ||
| 11.9 | FeH | ||||||
| 3.5 | Fe7C3 | ||||||
| 15 | 2000 | 24.9 | pyroxene glass | ||||
| 12.9 | new Fe3+ component | ||||||
| 36.5 | FeH | ||||||
| 25.6 | Fe7C3 | ||||||
| 16 | Crude oil + ferropericlase (Mg0.8Fe0.2)O | 9.5 | 1300 | 100.0 | ferropericlase | ||
| 17 | 1700 | 39.8 | ferropericlase | ||||
| 24.9 | FeH | ||||||
| 22.0 | Fe7C3a | ||||||
| 11.8 | Fe7C3b | ||||||
| 1.5 | α-Fe | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serovaiskii, A.; Mukhina, E.; Dubrovinsky, L.; Chernoutsan, A.; Kudryavtsev, D.; McCammon, C.; Aprilis, G.; Kupenko, I.; Chumakov, A.; Hanfland, M.; et al. Fate of Hydrocarbons in Iron-Bearing Mineral Environments during Subduction. Minerals 2019, 9, 651. https://doi.org/10.3390/min9110651
Serovaiskii A, Mukhina E, Dubrovinsky L, Chernoutsan A, Kudryavtsev D, McCammon C, Aprilis G, Kupenko I, Chumakov A, Hanfland M, et al. Fate of Hydrocarbons in Iron-Bearing Mineral Environments during Subduction. Minerals. 2019; 9(11):651. https://doi.org/10.3390/min9110651
Chicago/Turabian StyleSerovaiskii, Aleksandr, Elena Mukhina, Leonid Dubrovinsky, Aleksey Chernoutsan, Daniil Kudryavtsev, Catherine McCammon, Georgios Aprilis, Ilya Kupenko, Aleksandr Chumakov, Michael Hanfland, and et al. 2019. "Fate of Hydrocarbons in Iron-Bearing Mineral Environments during Subduction" Minerals 9, no. 11: 651. https://doi.org/10.3390/min9110651
APA StyleSerovaiskii, A., Mukhina, E., Dubrovinsky, L., Chernoutsan, A., Kudryavtsev, D., McCammon, C., Aprilis, G., Kupenko, I., Chumakov, A., Hanfland, M., & Kutcherov, V. (2019). Fate of Hydrocarbons in Iron-Bearing Mineral Environments during Subduction. Minerals, 9(11), 651. https://doi.org/10.3390/min9110651