Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle
Abstract
:1. Introduction
2. Methods and Procedures
2.1. Experimental Methods
2.2. Analytical Methods
2.3. PERPLE_X Calculation Strategy and Procedure
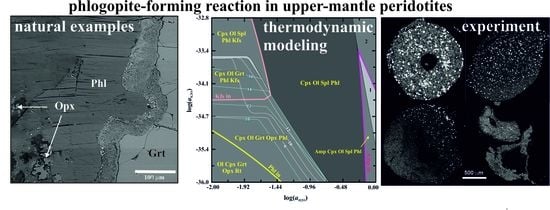
3. Phlogopite-Forming Reactions in the Upper-Mantle Rocks
3.1. Reactions in Peridotites
3.2. Experimental Studies of Phlogopite-Forming Reactions in Peridotites
3.3. Relation of Phlogopite with Other Potassic Phases in the Metasomatized Upper-Mantle Rocks
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd, F.E.; Bailey, D.K. Light element metasomatism of the continental mantle: The evidence and the consequences. Phys. Chem. Earth 1975, 9, 389–416. [Google Scholar] [CrossRef]
- Bailey, D.K. Mantle metasomatism—Continued chemical change within the earth. Nature 1982, 296, 525–580. [Google Scholar] [CrossRef]
- Bailey, D.K. Mantle metasomatism—Perspective and prospect. In Alkaline Igneous Rocks; Fitton, J.G., Upton, B.G.J., Eds.; Geological Society Special Publication: London, UK, 1987; Volume 30, pp. 1–13. [Google Scholar]
- Menzies, M.A.; Hawkesworth, C.J. Mantle Metasomatism; Academic Press: London, UK, 1987; p. 472. [Google Scholar]
- O’Reilly, S.Y.; Griffin, W.L. Mantle metasomatism. In Metasomatism and the Chemical Transformation of Rock; Harlov, D.E., Austerheim, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 471–533. [Google Scholar]
- Weiss, Y.; McNeill, J.; Pearson, D.G.; Nowell, G.M.; Ottley, C.J. Highly saline fluids from a subducting slab as the source for fluid-rich diamonds. Nature 2015, 524, 339. [Google Scholar] [CrossRef] [PubMed]
- Schiano, P.; Clocchiatti, R. Worldwide occurrence of silica-rich melts in sub-continental and sub-oceanic mantle minerals. Nature 1994, 368, 621–624. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Kamenetsky, V.S.; Korsakov, A.V.; Yaxley, G.M. Alkali-carbonate melts from the base of cratonic lithospheric mantle: Links to kimberlites. Chem. Geol. 2018, 483, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Förster, M.W.; Foley, S.F.; Marschall, H.R.; Alard, O.; Buhre, S. Melting of sediments in the deep mantle produces saline fluid inclusions in diamonds. Sci. Adv. 2019, 5, eaau2620. [Google Scholar] [CrossRef] [Green Version]
- Förster, M.W.; Prelević, D.; Buhre, S.; Mertz-Kraus, R.; Foley, S.F. An experimental study of the role of partial melts of sediments versus mantle melts in the sources of potassic magmatism. J. Asian Earth Sci. 2019, 177, 76–88. [Google Scholar] [CrossRef]
- Grassi, D.; Schmidt, M.W. Melting of carbonated pelites at 8–13 GPa: Generating K-rich carbonatites for mantle metasomatism. Contrib. Mineral. Petrol. 2011, 162, 169–191. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Schmidt, M.W. Melting of carbonated pelites at 2.5–5.0 GPa, silicate–carbonatite liquid immiscibility, and potassium–carbon metasomatism of the mantle. Earth Planet. Sci. Lett. 2008, 267, 17–31. [Google Scholar] [CrossRef]
- Perchuk, A.L.; Serdyuk, A.A.; Zinovieva, N.G. Subduction Sediment–Lherzolite Interaction at 2.9 GPa: Effects of Metasomatism and Partial Melting. Petrology 2019, 27, 467–488. [Google Scholar] [CrossRef]
- Gupta, A.K.; Fyfe, W.S. The Young Potassic Rocks; Ane Books: New Delhy, India, 2003; p. 370. [Google Scholar]
- Gupta, A.K. Origin of Potassium-Rich Silica-Deficient Igneous Rocks; Springer: New Delhy, India, 2015; p. 536. [Google Scholar]
- Harte, B. Mantle peridotites and processes—The kimberlite sample. In Continental Basalts and Mantle Xenoliths; Hawkesworth, C.J., Norry, M.J., Eds.; Shiva: Cheshire, UK, 1983; pp. 46–91. [Google Scholar]
- Yoder, H.S.; Kushiro, I. Melting of a hydrous phase: Phlogopite. Am. J. Sci. 1969, 267, 558–582. [Google Scholar]
- Trønnes, R.G. Stability range and decomposition of potassic richterite and phlogopite end members at 5–15 GPa. Mineral. Petrol. 2002, 74, 129–148. [Google Scholar] [CrossRef]
- Konzett, J.; Fei, Y. Transport and storage of potassium in the Earth’s upper mantle and transition zone: An experimental study to 23 GPa in simplified and natural bulk compositions. J. Petrol. 2000, 41, 583–603. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental study of the system phlogopite-diopside from 3.5 to 17 GPa. Am. Mineral. 1997, 82, 1198–1209. [Google Scholar] [CrossRef]
- Trønnes, R.G.; Edgar, A.D.; Arima, M. A high pressure-high temperature study of TiO2 solubility in Mg-rich phlogopite: Implications to phlogopite chemistry. Geochim. Cosmochim. Acta 1985, 49, 2323–2329. [Google Scholar] [CrossRef]
- Prinz, M.; Mansoni, D.V.; Hlava, P.F.; Keil, K. Inclusions in diamonds: Garnet lherzolite and eclogite assemblages. In Physics and Chemistry of the Earth; Elsevier: Amsterdam, The Netherlands, 1975; pp. 797–815. [Google Scholar]
- Sobolev, N.V.; Kaminsky, F.V.; Griffin, W.L.; Yefimova, E.S.; Win, T.T.; Ryan, C.G.; Botkunov, A.I. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Yefimova, E.S.; Channer, D.D.; Anderson, P.F.N.; Barron, K.M. Unusual upper mantle beneath Guaniamo, Guyana Shield, Venezuela: Evidence from diamond inclusions. Geology 1998, 26, 971–974. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Efimova, E.S. Syngenetic phlogopite inclusions in kimberlite-hosted diamonds: Implications for role of volatiles in diamond formation. Russ. Geol. Geophys. 2009, 50, 1234–1248. [Google Scholar] [CrossRef]
- Leost, I.; Stachel, T.; Brey, G.P.; Harris, J.W.; Ryabchikov, I.D. Diamond formation and source carbonation: Mineral associations in diamonds from Namibia. Contrib. Mineral. Petrol. 2003, 145, 15–24. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Zedgenizov, D.A.; Wirth, R. Specific multiphase assemblages of carbonatitic and Al-rich silicic diamond-forming fluids/melts: TEM observation of microinclusions in cuboid diamonds from the placers of Northeastern Siberian Craton. Minerals 2019, 9, 50. [Google Scholar] [CrossRef]
- Safonov, O.G.; Butvina, V.G. Indicator reactions of K and Na activities in the upper mantle: Natural mineral assemblages, experimental data, and thermodynamic modeling. Geochem. Intern. 2016, 54, 858–872. [Google Scholar] [CrossRef]
- Connolly, J.A.D. Computation of phase equilibria by linear programming: A tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 2005, 236, 524–541. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 2011, 29, 333–383. [Google Scholar]
- Jennings, E.S.; Holland, T.J. A simple thermodynamic model for melting of peridotite in the system NCFMASOCr. J. Petrol. 2015, 56, 869–892. [Google Scholar] [CrossRef]
- Green, E.C.R.; White, R.W.; Diener, J.F.A.; Powell, R.; Holland, T.J.B.; Palin, R.M. Activity–composition relations for the calculation of partial melting equilibria in metabasic rocks. J. Metamorph. Geol. 2016, 34, 845–869. [Google Scholar] [CrossRef]
- Tajčmanová, L.; Connolly, J.A.D.; Cesare, B. A thermodynamic model for titanium and ferric iron solution in biotite. J. Metamorph. Geol. 2009, 27, 153–165. [Google Scholar] [CrossRef]
- White, R.W.; Powell, R.; Holland, T.J.B.; Johnson, T.E.; Green, E.C.R. New mineral activity–composition relations for thermodynamic calculations in metapelitic systems. J. Metamorph. Geol. 2014, 32, 261–286. [Google Scholar] [CrossRef]
- Fuhrman, M.L.; Lindsley, D.H. Ternary-feldspar modeling and thermometry. Am. Mineral. 1988, 73, 201–215. [Google Scholar]
- Holland, T.J.B.; Powell, R. COmpensated Redlich-Kwong (CORK) fugacity equations for H2O and CO2. Contrib. Mineral. Petrol. 1991, 109, 265–273. [Google Scholar] [CrossRef]
- Carswell, D.A. Primary and secondary phlogopites and clinopyroxenes in garnet lherzolite xenoliths. Phys. Chem. Earth. 1975, 9, 417–429. [Google Scholar] [CrossRef]
- Jones, A.P.; Smith, J.V.; Dawson, J.B. Mantle metasomatism in 14 veined peridotites from Bultfontein Mine, South Africa. J. Geol. 1982, 90, 435–453. [Google Scholar] [CrossRef]
- Erlank, A.J.; Waters, F.G.; Hawkesworth, C.J.; Haggerty, S.E.; Allsopp, H.L.; Rickard, R.S.; Menzies, M.A. Evidence for mantle metasomatism in peridotite nodules from the Kimberley pipes, South Africa. In Mantle Metasomatism; Menzies, M.A., Hawkesworth, C.J., Eds.; Academic Press: London, UK, 1987; pp. 221–311. [Google Scholar]
- Waters, F.G.; Erlank, A.J. Assessment of the vertical extent and distribution of mantle metasomatism below Kimberley, South Africa. J. Petrol. 1988, 185–204. [Google Scholar] [CrossRef]
- Canil, D.; Scarfe, C.M. Origin of phlogopite in mantle xenoliths from Kostal Lake, Wells Gray Park, British Columbia. J. Petrol. 1989, 30, 1159–1179. [Google Scholar] [CrossRef]
- Lloyd, F.E.; Edgar, A.D.; Forsyth, D.M.; Barnett, R.L. The paragenesis of upper-mantle xenoliths from the Quaternary volcanics south-east of Gees, West Eifel, Germany. Mineral. Mag. 1991, 55, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Schulze, D.J. Low-Ca garnet harzburgites from Kimberley, South Africa: Abundance and bearing on the structure and evolution of the lithosphere. J. Geophys. Res. Solid Earth 1995, 100, 12513–12526. [Google Scholar] [CrossRef]
- Griffin, W.L.; Shee, S.R.; Ryan, C.G.; Win, T.T.; Wyatt, B.A. Harzburgite to lherzolite and back again: Metasomatic processes in ultramafic xenoliths from the Wesselton kimberlite, Kimberley, South Africa. Contrib. Mineral. Petrol. 1999, 134, 232–250. [Google Scholar] [CrossRef]
- Konzett, J.; Armstrong, R.A.; Günther, D. Modal metasomatism in the Kaapvaal craton lithosphere: Constraints on timing and genesis from U–Pb zircon dating of metasomatized peridotites and MARID-type xenoliths. Contrib. Mineral. Petrol 2000, 139, 704–719. [Google Scholar] [CrossRef]
- van Achterbergh, E.; Griffin, W.L.; Stiefenhofer, J. Metasomatism in mantle xenoliths from the Letlhakane kimberlites: Estimation of element fluxes. Contrib. Mineral. Petrol. 2001, 141, 397–414. [Google Scholar] [CrossRef]
- Kargin, A.V.; Sazonova, L.V.; Nosova, A.A.; Lebedeva, N.M.; Kostitsyn, Y.A.; Kovalchuk, E.V.; Tretyachenko, V.V.; Tikhomirova, Y.S. Phlogopite in mantle xenoliths and kimberlite from the Grib pipe, Arkhangelsk province, Russia: Evidence for multi-stage mantle metasomatism and origin of phlogopite in kimberlite. Geosci. Front. 2019, 10, 1941–1959. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Kushiro, I.; Aoki, K. Origin of some eclogite inclusions in kimberlite. Am. Mineral. 1968, 53, 1347–1367. [Google Scholar]
- Aoki, K. Origin of phlogopite and potassic richterite bearing peridotite xenoliths from South Africa. Contrib. Mineral. Petrol. 1975, 53, 145–156. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Ionov, D.A.; O’Reilly, S.Y.; Ashchepkov, I.V. Feldspar-bearing lherzolite xenoliths in alkali basalts from Hamar-Daban, southern Baikal region, Russia. Contrib. Mineral. Petrol. 1995, 122, 174–190. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kruk, A.N.; Chebotarev, D.A.; Pal’yanov, Y.N.; Sobolev, N.V. Conditions of phlogopite formation upon interaction of carbonate melts with peridotite of the subcratonic lithosphere. Doklady Earth Sci. 2015, 462, 638–642. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kruk, A.N.; Palyanov, Y.N.; Sobolev, N.V. Stability of phlogopite in ultrapotassic kimberlite-like systems at 5.5–7.5 GPa. Contrib. Mineral. Petrol. 2017, 172, 21. [Google Scholar] [CrossRef]
- Francis, D.M. The origin of amphibole in lherzolite xenoliths from Nunivak Island, Alaska. J. Petrol. 1976, 17, 357–378. [Google Scholar] [CrossRef]
- Delaney, J.S.; Smith, J.V.; Carswell, D.A.; Dawson, J.B. Chemistry of micas from kimberlites and xenoliths. II. Primary-secondary textured micas from peridotite xenoliths. Geochim. Cosmochim. Acta 1980, 44, 857–872. [Google Scholar] [CrossRef]
- Arai, S. K/Na variation in phlogopite and amphibole of upper mantle peridotites due to fractionation of the metasomatizing fluids. J. Geol. 1986, 94, 436–444. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H.; Kamenetsky, V.S. Carbonatite metasomatism in the southeastern Australian lithosphere. J. Petrol 1998, 39, 1917–1930. [Google Scholar] [CrossRef]
- Pirard, C.; Hermann, J. Experimentally determined stability of alkali amphibole in metasomatised dunite at sub-arc pressures. Contrib. Mineral. Petrol. 2015, 169, 1. [Google Scholar] [CrossRef]
- Safonov, O.G.; Butvina, V.G. Interaction of model peridotite with H2O-KCl fluid: Experiment at 1.9 GPa and its implications for upper mantle metasomatism. Petrology 2013, 21, 599–615. [Google Scholar] [CrossRef]
- Edgar, A.D.; Arima, M. Experimental studies on K-metasomatism of a model pyrolite mantle and their bearing on the genesis of uitrapotassic magmas. In Proceedings of the 27th International Geological Congress, Moscow, Russia, 4–14 August 1984; Volume 9, pp. 509–541. [Google Scholar]
- Thibault, Y.; Edgar, A.D. Patent mantle-metasomatism: Inferences based on experimental studies. Proc. Ind. Acad. Sci. Earth Planet. Sci. 1990, 99, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Ulmer, P.; Sweeney, R.J. Generation and differentiation of group II kimberlites: Constraints from a high-pressure experimental study to 10 GPa. Geochim. Cosmochim. Acta 2002, 66, 2139–2153. [Google Scholar] [CrossRef]
- Foley, S.F.; Yaxley, G.M.; Rosenthal, A.; Buhre, S.; Kiseeva, E.S.; Rapp, R.P.; Jacob, D.E. The composition of near-solidus melts of peridotite in the presence of CO2 and H2O between 40 and 60 kbar. Lithos 2009, 112, 274–283. [Google Scholar] [CrossRef]
- Enggist, A.; Chu, L.; Luth, R.W. Phase relations of phlogopite with magnesite from 4 to 8 GPa. Contrib. Mineral. Petrol. 2012, 163, 467–481. [Google Scholar] [CrossRef]
- Enggist, A.; Luth, R.W. Phase relations of phlogopite and pyroxene with magnesite from 4 to 8 GPa: KCMAS–H2O and KCMAS–H2O–CO2. Contrib. Mineral. Petrol. 2016, 171, 88. [Google Scholar] [CrossRef]
- Dawson, J.B.; Smith, J.V. The MARID (mica-amphibole-rutile-ilmenite-diopside) suite of xenoliths in kimberlite. Geochim. Cosmochim. Acta 1977, 41, 309–323. [Google Scholar] [CrossRef]
- Grégoire, M.; Bell, D.; Le Roex, A. Trace element geochemistry of phlogopite-rich mafic mantle xenoliths: Their classification and their relationship to phlogopite-bearing peridotites and kimberlites revisited. Contrib. Mineral. Petrol. 2002, 142, 603–625. [Google Scholar] [CrossRef]
- Kushiro, I.; Erlank, A.J. Stability of potassic richterite. Carnegie Inst. Wash. Yearb 1970, 68, 231–233. [Google Scholar]
- Sudo, A.; Tatsumi, Y. Phlogopite and K-amphibole in the upper mantle: Implication for magma genesis in subduction zones. Geophys. Res. Lett. 1990, 17, 29–32. [Google Scholar] [CrossRef]
- Konzett, J.; Sweeney, R.J.; Thompson, A.B.; Ulmer, P. Potassium amphibole stability in the upper mantle: An experimental study in a peralkaline KNCMASH system to 8.5 GPa. J. Petrol. 1997, 38, 537–568. [Google Scholar] [CrossRef]
- Konzett, J.; Ulmer, P. The stability of hydrous potassic phases in lherzolitic mantle—An experimental study to 9.5 GPa in simplified and natural bulk compositions. J. Petrol. 1999, 40, 629–652. [Google Scholar] [CrossRef]
- Yagi, A.; Suzuki, T.; Akaogi, M. High pressure transitions in the system KAlSi3O8-NaAlSi3O8. Phys. Chem. Minerals 1994, 21, 12–17. [Google Scholar] [CrossRef]
- Fasshauer, D.W.; Wunder, B.; Chatterjee, N.D.; Höhne, G.W. Heat capacity of wadeite-type K2Si4O9 and the pressure-induced stable decomposition of K-feldspar. Contrib. Mineral. Petrol. 1998, 131, 210–218. [Google Scholar] [CrossRef]
- Meyer, H.O.A.; McCallum, M.E. Mineral inclusions in diamonds from the Sloan kimberlites, Colorado. J. Geol. 1986, 94, 600–612. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Argunov, K.P. Inclusions of K-feldspar in diamond crystal from the “Mir” pipe. Doklady Akad. Nauk SSSR Earth Sci. 1985, 284, 953–956. [Google Scholar]
- Otter, M.; Gurney, J.J. Mineral inclusions in diamonds from the Sloan diatrems, Colorado-Wyoming state line kimberlite district, North America. In Kimberlites and Related Rocks. V.2 Their Crust/Mantle Setting: Diamonds and Diamond Exploration; Blackwell: Jiangxi, China, 1989; pp. 1042–1053. [Google Scholar]
- Novgorodov, P.G.; Bulanova, G.P.; Pavlova, L.A.; Mikhailov, V.N.; Ugarov, V.V.; Shebanin, A.P.; Argunov, K.P. Inclusions of potassic phases, coesite and omphacite in the coated diamond crystal from the “Mir” pipe. Doklady Akad. Nauk SSSR Earth Sci. 1990, 310, 439–443. [Google Scholar]
- Davies, R.M.; Griffin, W.L.; O’Reilly, S.Y.; Doyle, B.J. Mineral inclusions and geochemical characteristics of microdiamonds from the DO27, A154, A21, A418, DO18, DD17 and Ranch Lake kimberlites at Lac de Gras, Slave Craton, Canada. Lithos 2004, 77, 39–55. [Google Scholar] [CrossRef]
- Anand, M.; Taylor, L.A.; Misra, K.C.; Carlson, W.D.; Sobolev, N.V. Nature of diamonds in Yakutian eclogites: Views from eclogite tomography and mineral inclusions in diamonds. Lithos 2004, 77, 333–348. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Zedgenizov, D.A.; Ragozin, A.; Kalinina, V.V. Diamondiferous subcontinental lithospheric mantle of the northeastern Siberian Craton: Evidence from mineral inclusions in alluvial diamonds. Gondwana Res. 2015, 28, 106–120. [Google Scholar] [CrossRef]
- Smyth, J.R.; Hatton, C.J. A coesite-sanidine grospydite from the Roberts Victor kimberlite. Earth Planet. Sci. Lett. 1977, 34, 284–290. [Google Scholar] [CrossRef]
- Wohletz, K.H.; Smyth, J.R. Origin of a Roberts Victor sanidine-coesite grospydite: Thermodynamic considerations. In Kimberlites II: The Mantle and Crust-Mantle Relationships; Kornprobst, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 33–42. [Google Scholar]
- Schulze, D.J.; Helmstaedt, H. Coesite-sanidine eclogite from kimberlite: Products of mantle fractionation or subduction? J. Geol. 1988, 96, 435–443. [Google Scholar] [CrossRef]
- Schmickler, B.; Jacob, D.E.; Foley, S.F. Eclogite xenoliths from the Kuruman kimberlites, South Africa: Geochemical fingerprinting of deep subduction and cumulate processes. Lithos 2004, 75, 173–207. [Google Scholar] [CrossRef]
- Misra, K.C.; Anand, M.; Taylor, L.A.; Sobolev, N.V. Multi-stage metasomatism of diamondiferous eclogite xenoliths from the Udachnaya kimberlite pipe, Yakutia, Siberia. Contrib. Mineral. Petrol. 2004, 146, 696–714. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Ragozin, A.L.; Zedgenizov, D.A.; Mityukhin, S. Evidence for multistage evolution in a xenolith of diamond-bearing eclogite from the Udachnaya kimberlite pipe. Lithos 2008, 105, 289–300. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Griffin, W.L. Diamond formation during metasomatism of mantle eclogite by chloride-carbonate melt. Contrib. Mineral. Petrol. 2018, 173, 84. [Google Scholar] [CrossRef]
- Wang, W. Formation of diamond with mineral inclusions of “mixed” eclogite and peridotite paragenesis. Earth Planet. Sci. Lett. 1998, 160, 831–843. [Google Scholar] [CrossRef]
- Wendlandt, R.F.; Eggler, D.H. Stability of sanidine+forsterite and its bearing on the genesis of potassium magmas and the distribution of potassium in the upper mantle. Earth Planet. Sci. Lett. 1980, 51, 215–220. [Google Scholar] [CrossRef]
- Ionov, D.A.; Gregoire, M.; Prikhod’ko, V.S. Feldspar–Ti-oxide metasomatism in off-cratonic continental and oceanic upper mantle. Earth Planet. Sci. Lett. 1999, 165, 37–44. [Google Scholar] [CrossRef]
- Olafsson, M.; Eggler, D.H. Phase relations of amphibole, amphibole-carbonate and phlogopite-carbonate peridotite: Petrologic constraints on the asthenosphere. Earth Planet. Sci. Lett. 1983, 64, 305–315. [Google Scholar] [CrossRef]
- Haggerty, S.E. Oxide mineralogy of the upper mantle. In Oxide Minerals: Petrologic and Magnetic Significance; Lindsley, D.H., Ed.; Reviews in Mineralogy; De Gruyter: Berlin, Germany, 1991; Volume 25, pp. 355–416. [Google Scholar]
- Foley, S.; Höfer, H.; Brey, G. High-pressure synthesis of priderite and members of the lindsleyite-mathiasite and hawthorneite-yimengite series. Contrib. Mineral. Petrol. 1994, 117, 164–174. [Google Scholar] [CrossRef]
- Konzett, J.; Yang, H.; Frost, D.J. Phase relations and stability of magnetoplumbite-and crichtonite-series phases under upper-mantle P–T conditions: An experimental study to 15 GPa with implications for LILE metasomatism in the lithospheric mantle. J. Petrol. 2005, 46, 749–781. [Google Scholar] [CrossRef]
- Konzett, J.; Wirth, R.; Hauzenberger, C.; Whitehouse, M. Two episodes of fluid migration in the Kaapvaal Craton lithospheric mantle associated with Cretaceous kimberlite activity: Evidence from a harzburgite containing a unique assemblage of metasomatic zirconium-phases. Lithos 2013, 182, 165–184. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Butvina, V.G.; Vorobey, S.S.; Safonov, O.G.; Varlamov, D.A.; Bondarenko, G.V.; Shapovalov, Y.B. Experimental study of the formation of chromium-bearing priderite and yimengite as products of modal mantle metasomatism. Doklady Earth Sci. 2019, 486, 711–715. [Google Scholar] [CrossRef]
- Almeida, V.; Janasi, V.; Svisero, D.; Nannini, F. Mathiasite-loveringite and priderite in mantle xenoliths from the Alto Paranaíba Igneous Province, Brazil: Genesis and constraints on mantle metasomatism. Open Geosci. 2014, 6, 614–632. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Golovin, A.V.; Sharygin, I.S.; Giuliani, A.; Rodemann, T.; Spetsius, Z.V.; Kamenetsky, M. Djerfisherite in kimberlites and their xenoliths: Implications for kimberlite melt evolution. Contrib. Mineral. Petrol. 2019, 174, 8. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Litasov, K.D.; Sharygin, V.V.; Shatskiy, A.F.; Ohtani, E. Genesis of djerfisherite in kimberlite-hosted mantle xenoliths. In Proceedings of the Goldschmidt Conference, Yokohama, Japan, 26 June–1 July 2016; p. 2812. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safonov, O.; Butvina, V.; Limanov, E. Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle. Minerals 2019, 9, 685. https://doi.org/10.3390/min9110685
Safonov O, Butvina V, Limanov E. Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle. Minerals. 2019; 9(11):685. https://doi.org/10.3390/min9110685
Chicago/Turabian StyleSafonov, Oleg, Valentina Butvina, and Evgenii Limanov. 2019. "Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle" Minerals 9, no. 11: 685. https://doi.org/10.3390/min9110685
APA StyleSafonov, O., Butvina, V., & Limanov, E. (2019). Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle. Minerals, 9(11), 685. https://doi.org/10.3390/min9110685