Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Thermal Decomposition Kinetics
3. Results and Discussion
3.1. Characterization
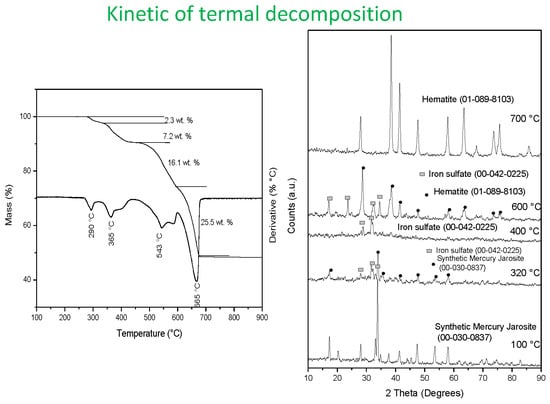
3.2. Thermal Characterization
3.3. Kinetics of Thermal Decomposition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barringer, L.I.; Szabo, Z.; Kauffman, L.J.; Barringer, T.H.; Stackelberg, P.E.; Ivahnenko, T.; Rajagopalan, S.; Krabbenhoft, D.P. Mercury concentrations in water from an unconfined aquifer system, New Jersey coastal plain. Sci. Total. Environ. 2005, 346, 169–183. [Google Scholar] [CrossRef]
- Borrell, A.; Aguilar, A.; Tornero, V.; Drago, M. Concentrations of mercury in tissues of striped dolphins suggest decline of pollution in Mediterranean open waters. Chemosphere 2014, 107, 319–323. [Google Scholar] [CrossRef]
- Counter, S.A.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef]
- Maurice-Bourgoin, L.; Quiroga, I.; Chincheros, J.; Courau, P. Mercury distribution in waters and fishes of the upper Madeira rivers and mercury exposure in riparian Amazonian populations. Sci. Total. Environ. 2000, 260, 73–86. [Google Scholar] [CrossRef]
- Crespo, M.E.; Macedo, G.L.; Pereira, S.I.D.; Arrifano, G.P.F.; Picanҫo, D.L.W.; Do Nascimento, J.L.M.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Wang, S.; Shang, L. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl. Geochem. 2005, 20, 627–638. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Qiu, G.; Wang, S.; Li, G.; Shang, L.; Meng, B.; Jiang, H.; Bai, W.; Li, Z. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ. Sci. Technol. 2007, 42, 326–332. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Wang, S.; Shang, L. Environmental contamination of mercury from Hg-mining areas in Wuchuan, northeastern Guizhou, China. Environ. Pollut. 2006, 142, 549–558. [Google Scholar] [CrossRef]
- Li, G.H.; Feng, X.B.; Qiu, G.L.; Bi, X.Y.; Li, Z.G.; Zhang, C.; Wang, D.Y.; Shang, L.H.; Guo, Y.N. Environmental mercury contamination of an artisanal zinc smelting area in Weining County, Guizhou, China. Environ. Pollut. 2008, 154, 21–31. [Google Scholar] [CrossRef]
- Garcia, A.; Murciego, A.; Alvarez, E.; Regina, I.S.; Rodriguez, M. Mercury in soils and plants in an abandoned cinnabar mining area (SW Spain). J. Hazard. Mater. 2009, 68, 1319–1324. [Google Scholar] [CrossRef]
- Pataranawat, P.; Parkpian, P.; Polprasert, C.; Delaune, R.; Jugsujinda, A. Mercury emission and distribution: Potential environmental risks at a small-scale gold mining operation, Phichit Province, Thailand. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2007, 42, 1081–1093. [Google Scholar] [CrossRef]
- Egler, S.G.; Rodrigues, S.; Villas, R.C.; Beinhoff, C. Evaluation of mercury pollution in cultivated and wild plants from two small communities of the Tapajos gold mining reserve, Para State, Brazil. Sci. Total Environ. 2006, 368, 424–433. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Farago, M. Mercury concentrations in a historic lead mining and smelting town in the Czech Republic: A pilot study. Sci. Total Environ. 1996, 188, 167–171. [Google Scholar] [CrossRef]
- Wang, J.; Fenga, X.; Andersonc, C.W.N.; Xingd, Y.; Shanga, L. Remediation of mercury contaminated sites —A review. J. Hazard. Mater. 2012, 221, 1–18. [Google Scholar] [CrossRef]
- Southworth, G.; Lindberg, S.; Zhang, H.; Anscombe, F. Fugitive mercury emissions from a chlor-alkali factory: Sources and fluxes to the atmosphere. Atmos. Environ. 2004, 38, 597–611. [Google Scholar] [CrossRef]
- Semu, E.; Singh, B.R.; Selmer-Olsen, A.R. Adsorption of mercury compounds by tropical soils II. Effect of soil: Solution ratio, ionic strength, pH, and organic matter. Water Air Soil Pollut. 1987, 32, 1–10. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Mercury in municipal solid waste in China and its control: A review. Environ. Sci. Technol. 2012, 46, 593–605. [Google Scholar] [CrossRef]
- Liu, J.; Valsaraj, K.T.; Devai, I.; DeLaune, R. Immobilization of aqueous Hg (II) by mackinawite (FeS). J. Hazard. Mater. 2008, 157, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Sun, H.W.; Yan, Q.S.; Sun, H.F. Laboratory study on remediation of mercury contaminated soil by iron chips. Ecol. Environ. 2007, 16, 437–441. (In Chinese) [Google Scholar]
- Backstrom, M.; Dario, M.; Karlsson, S.; Allard, B. Effects of a fulvic acid on the adsorption of mercury and cadmium on goethite. Sci. Total Environ. 2003, 304, 257–268. [Google Scholar] [CrossRef]
- Meng, X.; Hua, Z.; Dermatas, D.; Wang, W. Immobilization of mercury (II) in contaminated soil with used tire rubber. J. Hazard. Mater. 1998, 57, 231–241. [Google Scholar] [CrossRef]
- Hovsepyan, A.; Bonzongo, J.C.J. Aluminum drinking water treatment residuals (Al-WTRs) as sorbent for mercury: Implications for soil remediation. J. Hazard. Mater. 2009, 164, 73–80. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. An evaluation of technologies for the heavy metal remediation of dredged sediments. J. Hazard. Mater. 2001, 85, 145–163. [Google Scholar] [CrossRef]
- Xiong, Z.; He, F.; Zhao, D.; Barnett, M.O. Immobilization of mercury in sediment using stabilized iron sulfide nanoparticles. Water Res. 2009, 43, 5171–5179. [Google Scholar] [CrossRef]
- Tungittiplakorn, W.; Cohen, C.; Leonard, W. Engineered polymeric nanoparticles for bioremediation of hydrophobic contaminants. Environ. Sci. Technol. 2005, 39, 1354–1358. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 2000, 40, 405–452. [Google Scholar] [CrossRef]
- Buckby, T.; Black, S.; Coleman, M.L.; Hodson, M.E. Fe-sulphate-rich evaporative mineral precipitates from the Rio Tinto, southwest Spain. Mineral. Mag. 2003, 67, 263–278. [Google Scholar] [CrossRef]
- Das, G.K.; Anand, S.; Acharya, S.; Das, R.P. Preparation and decomposition of ammoniojarosite at elevated temperatures in H2O-(NH4)2SO4-H2SO4 media. Hydrometallurgy 1995, 38, 263–276. [Google Scholar] [CrossRef]
- Patiño, F.; Flores, M.U.; Reyes, I.A.; Reyes, M.; Hernández, J.; Rivera, I.; Juárez, J.C. Alkaline decomposition of synthetic jarosite with arsenic. Geochem. Trans. 2013, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Patiño, F.; Reyes, I.A.; Flores, M.U.; Pandiyan, T.; Roca, A.; Reyes, M.; Hernández, J. Kinetic modeling and experimental design of the sodium arsenojarosite decomposition in alkaline media: Implications. Hydrometallurgy 2013, 137, 115–125. [Google Scholar] [CrossRef]
- Flores, M.U.; Patiño, F.; Reyes, I.A.; Reyes, M.; Rivera, I.; Juárez, J.C. Kinetic modeling of the alkaline decomposition of potassium arsenojarosite. J. Braz. Chem. Soc. 2012, 23, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Reyes, I.A.; Patiño, F.; Rivera, I.; Flores, M.U.; Reyes, M.; Hernández, J. Alkaline reactivity of arsenical natrojarosite. J Braz Chem Soc. 2012, 22, 2260–2267. [Google Scholar] [CrossRef]
- Asta, M.P.; Cama, J.; Martínez, M.; Giménez, J. Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J. Hazard. Mater. 2009, 171, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Dutrizac, J.E.; Kaiman, S. Synthesis and properties of jarosite-type compounds. Can. Mineral. 1976, 14, 151–158. [Google Scholar]
- Dutrizac, J.E.; Chen, T.T. The synthesis of mercury jarosite and the mercury concentration in jarosite-family minerals. Can. Mineral. 1981, 19, 550–569. [Google Scholar]
- Frost, R.L.; Weier, M.L.; Martens, W. Thermal decomposition of jarosites of potassium, sodium and lead. J. Therm. Anal. Calorim. 2005, 82, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Wills, R.A.; Kloprogge, J.T.; Materns, W.N. Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 83, 213–218. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.A.; Kloprogge, J.T.; Materns, W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 84, 489–496. [Google Scholar] [CrossRef]
- Ordoñez, S.; Patiño, F.; Reyes, I.A.; Flores, M.U.; Flores, V.H.; Palacios, E.G.; Reyes, M. Synthesis and topology of the reaction of mercury jarosite in NaOH medium. In Proceedings of the European Metallurgical Conference, Duesseldorf, Germany, 14–17 June 2015; Volume 1, pp. 537–552. [Google Scholar]
- Jeffery, G.H.; Basset, J.; Mendham, J.; Denney, R.C. Vogel’s, Textbook of Quantitative Chemical Analysis; Longman Scientific and Technical: London, UK, 1989. [Google Scholar]
- Askarinejad, A.; Morsali, A. Synthesis and characterization of mercury oxide unusual nanostructures by ultrasonic method. Chem. Eng. J. 2009, 153, 183–186. [Google Scholar] [CrossRef]
- Xhaxhiu, K.; Saraҫi, E.; Bente, K. Sequestration of supercritical CO2 by mercury oxide. Chem. Pap. 2013, 67, 594–600. [Google Scholar] [CrossRef]
- Refat, M.S.; Elsabawy, K.M. Infrared spectra, Raman laser, XRD, DSC/TGA and SEM investigations on the preparations of selenium metal, (Sb2O3, Ga2O3, SnO and HgO) oxides and lead carbonate with pure grade using acetamide precursors. Bull. Mater. Sci. 2011, 34, 873–881. [Google Scholar] [CrossRef]
- Bishop, J.L.; Murad, E. The visible and infrared spectral properties of jarosite and alunite. Am. Mineral. 2005, 90, 1100–1107. [Google Scholar] [CrossRef]
- Sasaki, K.; Tanaike, O.; Konno, H. Distinction of jarosite-group compounds by Raman spectroscopy. Can. Mineral. 1998, 36, 1225–1235. [Google Scholar]
- Jiménez, A.; Prieto, M. Thermal stability of ettringite exposed to atmosphere: Implications for the uptake of harmful ions by cement. Environ. Sci. Technol. 2015, 49, 7957–7964. [Google Scholar] [CrossRef]
- Putnis, A. Introduction to Mineral Sciences; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Ballester, A.; Felipe Verdeja, L.; Sancho, J. Metalurgia Extractiva, 2nd ed.; Síntesis: Barcelona, Spain, 2000; Volume I. [Google Scholar]










| Specie | wt. % | Analysis Technique |
|---|---|---|
| Fe | 26.9 | AAS |
| SO4 | 37.1 | Gravimetric |
| Hg | 14.1 | AAS |
| OH + H3O + H2O | 21.9 | Difference |
| Spectral Band (cm−1) | Related Vibration | Spectral Band (cm−1) | Related Vibration |
|---|---|---|---|
| 3310 vb | νOH | 671 vw | ν4SO42− |
| 2350–1900 w, b | 2νSO42−, 2δOH− | 636 w | ν4SO42− |
| 1630 w | δH2O | 618 m | Hg–O |
| 1184 s | ν3SO42− | 563 sh | γOH− |
| 1087 s | ν3SO42− | 490 sh | νFe–O, νHg–O |
| 1004 vs | δOH− | 465 s | νFe–O, νHg–O |
| 996 sh | ν1SO42− | 430 s | ν2SO42− |
| Stage | Time to 50%, tY (min) | ln (tY) | Temperature (°C) | 1000/T (K−1) | Ea (kJ∙mol−1 ) |
|---|---|---|---|---|---|
| First | 3.1 | 1.13 | 290 | 1.77 | 50.80 |
| 2.37 | 0.86 | 305 | 1.73 | ||
| 2.1 | 0.74 | 315 | 1.70 | ||
| 1.6 | 0.47 | 325 | 1.67 | ||
| Second | 2.3 | 0.83 | 360 | 1.57 | 20.19 |
| 2.1 | 0.74 | 375 | 1.54 | ||
| 2 | 0.69 | 390 | 1.50 | ||
| 1.8 | 0.58 | 400 | 1.48 | ||
| Third | 2.3 | 0.83 | 520 | 1.26 | 44.80 |
| 2.1 | 0.74 | 545 | 1.22 | ||
| 1.7 | 0.54 | 560 | 1.20 | ||
| 1.5 | 0.40 | 590 | 1.15 | ||
| Fourth | 2.42 | 0.88 | 600 | 1.14 | 234.70 |
| 1.45 | 0.37 | 620 | 1.11 | ||
| 1.03 | 0.03 | 635 | 1.10 | ||
| 0.38 | −0.96 | 650 | 1.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, M.U.; Reyes, I.A.; Palacios, E.G.; Patiño, F.; Juárez, J.C.; Reyes, M.; Teja, A.M.; Islas, H.; Gutiérrez, E.J. Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite. Minerals 2019, 9, 200. https://doi.org/10.3390/min9040200
Flores MU, Reyes IA, Palacios EG, Patiño F, Juárez JC, Reyes M, Teja AM, Islas H, Gutiérrez EJ. Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite. Minerals. 2019; 9(4):200. https://doi.org/10.3390/min9040200
Chicago/Turabian StyleFlores, Mizraim U., Iván A. Reyes, Elia G. Palacios, Francisco Patiño, Julio C. Juárez, Martín Reyes, Aislinn M. Teja, Hernán Islas, and Emmanuel J. Gutiérrez. 2019. "Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite" Minerals 9, no. 4: 200. https://doi.org/10.3390/min9040200
APA StyleFlores, M. U., Reyes, I. A., Palacios, E. G., Patiño, F., Juárez, J. C., Reyes, M., Teja, A. M., Islas, H., & Gutiérrez, E. J. (2019). Kinetic Analysis of the Thermal Decomposition of a Synthetic Mercury Jarosite. Minerals, 9(4), 200. https://doi.org/10.3390/min9040200