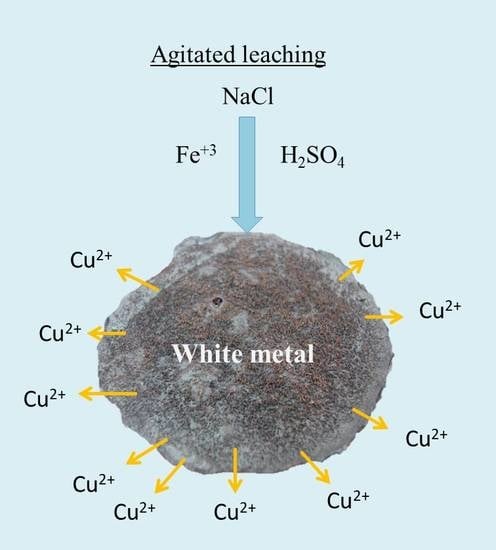
Leaching of White Metal in a NaCl-H2SO4 System under Environmental Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. White Metal Characterization
2.2. Leaching Tests
3. Results and Discussion
3.1. Baseline
3.2. Effect of Ferric Ion
3.3. Effect of Strong Chloride Media
3.4. Effect of Sulfuric Acid
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.-M.; Guo, X.-Y.; Wang, S.-S.; Liao, L.-L.; Tian, Q.-H. Multiphase equilibrium modeling of oxygen bottom-blown copper smelting process. Trans. Nonferrous Met. Soc. China 2017, 27, 2503–2511. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X. Thermodynamic Modeling of Oxygen Bottom-Blowing Continuous Converting Process. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 573–583. [Google Scholar]
- Hogg, B.; Nikolic, S.; Voigt, P.; Telford, P. ISASMELT Technology for Sulfide Smelting. In Extraction 2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–158. [Google Scholar]
- Ruiz, M.C.; Gallardo, E.; Padilla, R. Copper extraction from white metal by pressure leaching in H2SO4-FeSO4-O2. Hydrometallurgy 2009, 100, 50–55. [Google Scholar] [CrossRef]
- Park, K.H.; Mohapatra, D.; Hong-In, K.; Xueyi, G. Dissolution behavior of a complex Cu-Ni-Co-Fe matte in CuCl2-NaCl-HCl leaching medium. Sep. Purif. Technol. 2007, 56, 303–310. [Google Scholar] [CrossRef]
- Anand, S.; Das, R.P.; Jena, P.K. Sulphuric acid pressure leaching of CuNiCo matte obtained from copper converter slag—Optimisation through factorial design. Hydrometallurgy 1991, 26, 379–388. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Viñals, J. Dissolution kinetics of copper, white metal and natural chalcocite in Cl2/Cl- media. Hydrometallurgy 1999, 51, 345–357. [Google Scholar] [CrossRef]
- Neustroev, V.I.; Karimov, K.A.; Naboichenko, S.S.; Kovyazin, A.A. Autoclave leaching of arsenic from copper concentrate and matte. Metallurgist 2015, 59, 177–179. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Abarzúa, E.; Padilla, R. Oxygen pressure leaching of white metal. Hydrometallurgy 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Padilla, R.; Pavez, P.; Ruiz, M.C. Kinetics of copper dissolution from sulfidized chalcopyrite at high pressures in H2SO4-O2. Hydrometallurgy 2008, 91, 113–120. [Google Scholar] [CrossRef]
- Watling, H.R.; Shiers, D.W.; Li, J.; Chapman, N.M.; Douglas, G.B. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51. [Google Scholar] [CrossRef]
- Arce, E.M.; González, I. A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution. Int. J. Miner. Process. 2002, 67, 17–28. [Google Scholar] [CrossRef]
- Miki, H.; Nicol, M.; Velásquez-Yévenes, L. The kinetics of dissolution of synthetic covellite, chalcocite and digenite in dilute chloride solutions at ambient temperatures. Hydrometallurgy 2011, 105, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, J.C.; Park, S.W.; Jeong, J.; Kumar, V. Dissolution behaviour of platinum by electro-generated chlorine in hydrochloric acid solution. J. Chem. Technol. Biotechnol. 2013, 88, 1212–1219. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroz, R.; Manzano, E.; Bou, C.; Viñals, J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy 1998, 49, 87–101. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Pilone, D.; Kelsall, G.H. Prediction and measurement of multi-metal electrodeposition rates and efficiencies in aqueous acidic chloride media. Electrochim. Acta 2006, 51, 3802–3808. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.-S.; Lee, J.-C.; Yoo, K.; Jeong, J. Leaching behavior of copper using electro-generated chlorine in hydrochloric acid solution. Hydrometallurgy 2010, 100, 95–102. [Google Scholar] [CrossRef]
- Padilla, R.; Girón, D.; Ruiz, M.C. Leaching of enargite in H2SO4-NaCl-O2 media. Hydrometallurgy 2005, 80, 272–279. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Jeong, J.; Pandey, B.D. Leaching kinetics of copper from waste printed circuit boards by electro-generated chlorine in HCl solution. Hydrometallurgy 2011, 107, 124–132. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.W.; Lee, J.C.; Choubey, P.K. A novel zero emission concept for electrogenerated chlorine leaching and its application to extraction of platinum group metals from spent automotive catalyst. Hydrometallurgy 2016, 159, 19–27. [Google Scholar] [CrossRef]
- Kleiv, R.; Aasly, K.; Kowalczuk, P.; Snook, B.; Drivenes, K.; Manaig, D. Galvanic leaching of seafloor massive sulphides using MnO2 in H2SO4-NaCl media. Minerals 2018, 8, 235. [Google Scholar]
- Beşe, A.V.; Ata, O.N.; Çelik, C.; Çolak, S. Determination of the optimum conditions of dissolution of copper in converter slag with chlorine gas in aqueous media. Chem. Eng. Process. 2003, 42, 291–298. [Google Scholar] [CrossRef]
- Fisher, W.W.; Flores, F.A.; Henderson, J.A. Comparison of chalcocite dissolution in the oxygenated, aqueous sulfate and chloride systems. Miner. Eng. 1992, 5, 817–834. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Chen, M. Investigation of Cu-S intermediate species during electrochemical dissolution and bioleaching of chalcopyrite concentrate. Hydrometallurgy 2013, 134–135, 158–165. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Torres, C.M.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Torres, C.M.; Taboada, M.E.; Graber, T.A.; Herreros, O.O.; Ghorbani, Y.; Watling, H.R. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Hernández, P.; Taboada, M.; Herreros, O.; Graber, T.; Ghorbani, Y. Leaching of chalcopyrite in acidified nitrate using seawater-based media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Salinas, K.; Herreros, O.; Torres, C. Leaching of primary copper sulfide ore in chloride-ferrous media. Minerals 2018, 8, 312. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Veloso, T.C.; Peixoto, J.J.M.; Pereira, M.S.; Leao, V.A. Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl. Int. J. Miner. Process. 2016, 148, 147–154. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Deniz Turan, M.; Boyrazlı, M.; Soner Altundoğan, H. Improving of copper extraction from chalcopyrite by using NaCl. J. Cent. South Univ. 2018, 25, 21–28. [Google Scholar]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(II)-OH-Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Zhou, K.; Pan, L.; Peng, C.; He, D.; Chen, W. Selective precipitation of Cu in manganese-copper chloride leaching liquor. Hydrometallurgy 2018, 175, 319–325. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Senanayake, G. A review of chloride assisted copper sulfide leaching by oxygenated sulfuric acid and mechanistic considerations. Hydrometallurgy 2009, 98, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.D.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with LIX 63 and Alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]
- Lawson, F.; Chu-Yong, C.; Ying Lee, S. Leaching of copper sulphides and copper mattes in oxygenated chloride/sulphate leachants. Miner. Process. Extr. Metall. Rev. 1992, 8, 183–203. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.Y.; Jeffrey, M.I.; Lawson, F. Effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Cheng, C.Y. A synergistic solvent extraction system for separating copper from iron in high chloride concentration solutions. Hydrometallurgy 2012, 113–114, 155–159. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Two-stage countercurrent solvent extraction of copper from cuprous chloride solution: Cu(II) loading coupled with Cu(I) oxidation by oxygen and iron scrubbing. Hydrometallurgy 2014, 150, 41–46. [Google Scholar] [CrossRef]










| Low Cl− | High Cl− | |||||
|---|---|---|---|---|---|---|
| Cu(II) | Cu2+ | CuCl+ | CuCl2 | CuCl3− | CuCl42− | |
| Cu(I) | CuCl2− | CuCl32− | CuCl43− | |||
| Fe(III) | Fe3+ | FeCl2+ | FeCl2+ | |||
| Fe(II) | Fe2+ | FeCl+ |
| H2SO4, g/L | 10 | 15 | 20 | 30 | 50 |
|---|---|---|---|---|---|
| %Fe | 3.14 | 2.99 | 2.67 | 2.35 | 2.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, J.; Sepúlveda, R.; Araya, G.; Guzmán, D.; Toro, N.; Pérez, K.; Rodríguez, M.; Navarra, A. Leaching of White Metal in a NaCl-H2SO4 System under Environmental Conditions. Minerals 2019, 9, 319. https://doi.org/10.3390/min9050319
Castillo J, Sepúlveda R, Araya G, Guzmán D, Toro N, Pérez K, Rodríguez M, Navarra A. Leaching of White Metal in a NaCl-H2SO4 System under Environmental Conditions. Minerals. 2019; 9(5):319. https://doi.org/10.3390/min9050319
Chicago/Turabian StyleCastillo, Jonathan, Rossana Sepúlveda, Giselle Araya, Danny Guzmán, Norman Toro, Kevin Pérez, Marcelo Rodríguez, and Alessandro Navarra. 2019. "Leaching of White Metal in a NaCl-H2SO4 System under Environmental Conditions" Minerals 9, no. 5: 319. https://doi.org/10.3390/min9050319
APA StyleCastillo, J., Sepúlveda, R., Araya, G., Guzmán, D., Toro, N., Pérez, K., Rodríguez, M., & Navarra, A. (2019). Leaching of White Metal in a NaCl-H2SO4 System under Environmental Conditions. Minerals, 9(5), 319. https://doi.org/10.3390/min9050319