Reuse of Dunite Mining Waste and Subproducts for the Stabilization of Metal(oid)s in Polluted Soils
Abstract
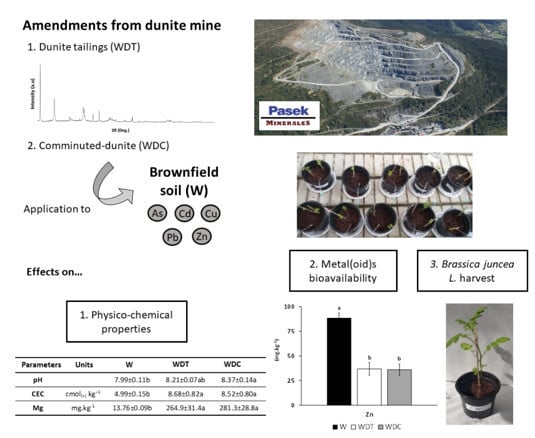
:1. Introduction
2. Material and Methods
2.1. Polluted Soil Sampling
2.2. Amendments
2.3. Greenhouse Experiment
2.4. Statistical Analysis
3. Results
3.1. Properties of the Soil and Amendments
3.2. Effects of the Amendments on the Physico-Chemical Properties of the Soil
3.3. Effects of the Amendments on Pollutants
3.4. Harvested Biomass of Brassica juncea L.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags–A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.C.; Mo, K.H.; Shi, C. A critical review of waste glass powder-Multiple roles of utilization in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.; McLaughlin, E.; Börger, T. The Circular Economy: Swings and Roundabouts? Ecol. Econ. 2019, 158, 11–19. [Google Scholar] [CrossRef]
- Rees, W. What’s blocking sustainability? Human nature, cognition, and denial. Sustain. Sci. Pract. Policy 2010, 6, 13–25. [Google Scholar] [CrossRef]
- Vlek, C.; Steg, L. Human behavior and environmental sustainability: Problems, driving forces and research topics. J. Soc. Issues 2007, 63, 1–19. [Google Scholar] [CrossRef]
- European Commission. EU SCIENCE HUB. The European Commission’s Science and Knowledge Service. Available online: https://ec.europa.eu/jrc/en/research-topic/waste-and-recycling (accessed on 5 July 2019).
- European Commission. Circular Economy. Closing the Loop. An Ambitious EU Circular Economy Package. 2019. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 5 July 2019).
- Velenturf, A.P.M.; Archer, S.A.; Gomes, H.I.; Christgen, B.; Lag-Brotons, J.; Purnell, P. Circular economy and the matter of integrated resources. Sci. Total Environ. 2019, 689, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine wastes: Past, present, future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- González-Fernández, B.; Rodríguez-Valdés, E.; Boente, C.; Menéndez-Casares, E.; Fernández-Braña, A.; Gallego, J.R. Long-term ongoing impact of arsenic contamination on the environmental compartments of a former mining-metallurgy area. Sci. Total Environ. 2018, 610–611, 820–830. [Google Scholar] [CrossRef]
- Clemente, R.; Arco-Lázaro, E.; Pardo, T.; Martín, I.; Sánchez-Guerrero, A.; Sevilla, F.; Bernal, M.P. Combination of soil organic and inorganic amendments helps plants overcome trace element induced oxidative stress and allows phytostabilisation. Chemosphere 2019, 223, 23–231. [Google Scholar] [CrossRef]
- Delil, A.D.; Köleli, N. Investigation of a combined continuous flow system for the removal of Pb and Cd from heavily contaminated soil. Chemosphere 2019, 229, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Pamukcu, S.; Shrestha, R.A. Simultaneous removal of Pb, Cd, and Zn from heavily contaminated mine tailing soil using enhanced electrochemical process. Environ. Eng. Sci. 2015, 32, 416–424. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments-An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Parke, J.; Makino, T.; Kirkham, M.B.; Shceckel, K. Remediation of heavy metal(loid)s contaminated soils-To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Del Valle-Zermeño, R.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Low-grade magnesium oxide by-products for environmental solutions: Characterization and geochemical performance. J. Geochem. Explor. 2015, 152, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Kayser, C.; Larkin, T.; Singhal, N. Amendment of biosolids with waste materials and lime: Effect on geoenvironmental properties and leachate production. Waste Manag. 2015, 46, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Porta, J.; Loόpez-Acevedo, M.; Rodriíguez, R. Técnicas y Experimentos en Edafología; Collegi Oficial D’Enginyers Agronoms de Catalunya: Barcelona, Spain, 1986. [Google Scholar]
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; VanVark, W. Soil analysis procedures using 0,01M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 3, 1299–1396. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- Rollinson, H. Dunites in the mantle section of the Oman ophiolite-The boninite connection. Lithos 2019, 334–335, 1–7. [Google Scholar] [CrossRef]
- Abily, B.; Geuleneer, G. The dunitic mantle-crust transition zone in the Oman ophiolite: Residue of melt-rock interaction, cumulates from high-MgO melts or both? Geology 2013, 41, 67–70. [Google Scholar] [CrossRef]
- Caballero, R.; García-Arias, M.; Rubio, L.; Corretgé, L.G. Dunite-A cost effective raw material in basic refractory mixes for Steel making. In Proceedings of the 52nd International Colloquium on Refractories, EUROGRESS, Aachen, Germany, 23–24 September 2009. [Google Scholar]
- Lessovaia, S.; Dultz, S.; Polekhovsky, Y.; Krupskaya, Y.; Vigasina, M.; Melchakova, L. Rock control of pedogenic clay mineral formation in a shallow soil from serpentinous dunite in the Polar Urals, Russia. Appl. Clay Sci. 2012, 64, 4–11. [Google Scholar] [CrossRef]
- Lessovaia, S.; Dultz, S.; Plötze, M.; Andreeva, N.; Polekhovsky, Y.; Filimonov, A.; Momotova, O. Soil development on basic and ultrabasic rocks in cold environments of Russia traced by mineralogical composition and pore space characteristics. Catena 2016, 137, 596–604. [Google Scholar] [CrossRef]
- Van Noort, R.; Mørkved, P.T.; Dundas, S.H. Acid neutralization by mining waste dissolution under conditions relevant for agricultural applications. Geosciences 2018, 8, 380. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B. Interpreting Soils Test Resulting. What Do All the Numbers Mean? CSIRO: Collingwood, VIC, Australia, 2007. [Google Scholar]
- Brewer, C.E.; Urger, U.; Schmidt-Rohr, K.; Brown, R.C. Criteria to Select Biochars for Field Studies based on Biochar Chemical Properties. BioEnergy Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Weng, L.; Temminghoff, E.J.M.; Van Riemsdijk, W.H. Contribution of individual sorbents to the control of heavy metal activity in sandy soil. Int. J. Environ. Sci. Technol. 2001, 35, 4436–4443. [Google Scholar] [CrossRef]
- Brammer, H.; Ravenscroft, P. Arsenic in groundwater: A threat to sustainable agriculture in South and South-east Asia. Environ. Int. 2009, 35, 647–654. [Google Scholar] [CrossRef]
- O’Neill, P. Arsenic. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie: London, UK, 1990; pp. 83–99. [Google Scholar]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Yan, X.P.; Kerrich, R.; Hendry, M.J. Distribution of arsenic(III), arsenic(V) and total inorganic arsenic in pore waters from a thick till and clay-rich aquitard sequence, Saskatchewan, Canada. Geochim. Cosmochim. Acta 2000, 62, 2637–2648. [Google Scholar] [CrossRef]
- Dudka, S.; Adriano, D.C. Environmental impacts of metal ore mining and processing: A review. J. Environ. Qual. 1997, 26, 590–602. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Clemente, R.; Lepp, N.; Dickinson, N. Mobility of arsenic, cadmium and zinc in a multi-element contaminated soil profile assessed by in-situ soil pore water sampling, column leaching and sequential extraction. Environ. Pollut. 2010, 158, 155–160. [Google Scholar] [CrossRef]
- Smith, S.R. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Forján, R.; Asensio, V.; Rodríguez-Vila, A.; Covelo, E.F. Contribution of waste and biochar amendment to the sorption of metals in a copper mine tailing. Catena 2016, 137, 120–125. [Google Scholar]
- Rieuwerts, J.; Ashmore, M.; Farago, M.; Thornton, I. The influence of soil characteristics on the extractability of Cd, Pb and Zn in upland and moorland soils. Sci. Total Environ. 2006, 366, 864–875. [Google Scholar] [CrossRef]
- Forján, R.; Rodríguez-Vila, R.; Cerqueira, B.; Covelo, E.F.; Marcet, P.; Asensio, V. Comparative effect of compost and technosol enhanced with biochar on the fertility of a degraded soil. Environ. Monit. Assess. 2018, 190, 610. [Google Scholar]
- Rodríguez-Vila, A.; Forján, R.; Guedes, R.S.F.; Covelo, E. Changes on the Phytoavailability of Nutrients in a Mine Soil Reclaimed with Compost and Biochar. Water Air Soil Pollut. 2016, 227, 453. [Google Scholar] [CrossRef]





| Code | Polluted Soil | Dunite-Tailings | Dunite-Comminuted | Brassica juncea L. |
|---|---|---|---|---|
| W | 100% | yes | ||
| WDT | 80% | 20% | yes | |
| WDC | 80% | 20% | yes |
| Parameters | Units | Samples | |||
|---|---|---|---|---|---|
| W | DT | DC | |||
| Edaphic | pH | 7.99 | 9.23 | 9.16 | |
| TC | mg·kg−1 | 23.10 | 5.31 | 2.20 | |
| TN | <DL | <DL | <DL | ||
| P | 3.14 | 2.21 | <2.00 | ||
| K | 25.75 | 78.60 | 42.55 | ||
| Mg | 27.17 | 577.70 | 672.50 | ||
| Ca | 466.70 | 120.10 | 143.90 | ||
| Na | 3.09 | 20.99 | 24.70 | ||
| CEC | cmol(+)·kg−1 | 5.30 | 11.30 | 13.00 | |
| Pseudo-total concentration | Cu | mg·kg−1 | 1707.80 | 28.78 | 33.84 |
| Zn | 3739.50 | 37.63 | 261.6 | ||
| Cd | 24.61 | 0.89 | 2.53 | ||
| As | 632.40 | 15.94 | 4.04 | ||
| Pb | 5112.20 | 17.71 | 47.98 | ||
| Available concentration | Cu | mg·kg−1 | 4.70 | <DL | 0.15 |
| Zn | 176.54 | <DL | 47.41 | ||
| Cd | 0.67 | <DL | 1.03 | ||
| As | 0.32 | <DL | 0.09 | ||
| Pb | 24.63 | 0.06 | 2.09 | ||
| Amendment | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Others | L.O.I |
|---|---|---|---|---|---|---|---|---|
| DT | 57.11 | 3.17 | 4.97 | 20.60 | 2.68 | 0.23 | 0.25 | 9.91 |
| DC | 39.86 | 3.00 | 7.62 | 35.34 | 1.73 | 0.07 | 0.35 | 11.91 |
| Parameters | Units | W | WDT | WDC |
|---|---|---|---|---|
| pH | 7.99 ± 0.11b | 8.21 ± 0.07ab | 8.37 ± 0.14a | |
| TC | mg·kg−1 | 22.6 ± 2.05a | 18.5 ± 1.17b | 18.6 ± 1.94b |
| TN | <DL | <DL | <DL | |
| CEC | cmol( + ) kg−1 | 4.99 ± 0.15b | 8.68 ± 0.82a | 8.52 ± 0.80a |
| Ca | mg·kg−1 | 447.9 ± 13.0a | 392.29 ± 29.5ab | 348.8 ± 29.9b |
| K | 22.50 ± 1.38a | 43.76 ± 5.22b | 40.14 ± 12.7b | |
| Mg | 13.76 ± 0.09b | 264.9 ± 31.4a | 281.3 ± 28.8a | |
| Na | 14.75 ± 1.79a | 14.11 ± 0.77a | 15.25 ± 2.95a | |
| P | 3.13 ± 0.75a | 2.00 ± 0.52a | 2.06 ± 0.09a |
| Metal(oid)s | Units | W | WDT | WDC |
|---|---|---|---|---|
| As | mg·kg−1 | 688.4 ± 19.6a | 469.1 ± 4.4b | 497.0 ± 34.7b |
| Cd | 25.5 ± 0.1a | 18.8 ± 0.3b | 19.3 ± 1.3b | |
| Cu | 1953.5 ± 2.37a | 1298.2 ± 47.3b | 1343.9 ± 85.5b | |
| Pb | 5564.2 ± 38.6a | 3881.3 ± 152.6b | 4051.8 ± 175.6b | |
| Zn | 3763.4 ± 48.5a | 2679.4 ± 43.0b | 2781.6 ± 137.3b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baragaño, D.; Forján, R.; Menéndez Aguado, J.M.; Covián Martino, M.; Díaz García, P.; Martínez Rubio, J.; Álvarez Rueda, J.J.; R. Gallego, J.L. Reuse of Dunite Mining Waste and Subproducts for the Stabilization of Metal(oid)s in Polluted Soils. Minerals 2019, 9, 481. https://doi.org/10.3390/min9080481
Baragaño D, Forján R, Menéndez Aguado JM, Covián Martino M, Díaz García P, Martínez Rubio J, Álvarez Rueda JJ, R. Gallego JL. Reuse of Dunite Mining Waste and Subproducts for the Stabilization of Metal(oid)s in Polluted Soils. Minerals. 2019; 9(8):481. https://doi.org/10.3390/min9080481
Chicago/Turabian StyleBaragaño, Diego, Rubén Forján, Juan María Menéndez Aguado, Marcos Covián Martino, Pamela Díaz García, Javier Martínez Rubio, Juan José Álvarez Rueda, and José Luis R. Gallego. 2019. "Reuse of Dunite Mining Waste and Subproducts for the Stabilization of Metal(oid)s in Polluted Soils" Minerals 9, no. 8: 481. https://doi.org/10.3390/min9080481
APA StyleBaragaño, D., Forján, R., Menéndez Aguado, J. M., Covián Martino, M., Díaz García, P., Martínez Rubio, J., Álvarez Rueda, J. J., & R. Gallego, J. L. (2019). Reuse of Dunite Mining Waste and Subproducts for the Stabilization of Metal(oid)s in Polluted Soils. Minerals, 9(8), 481. https://doi.org/10.3390/min9080481