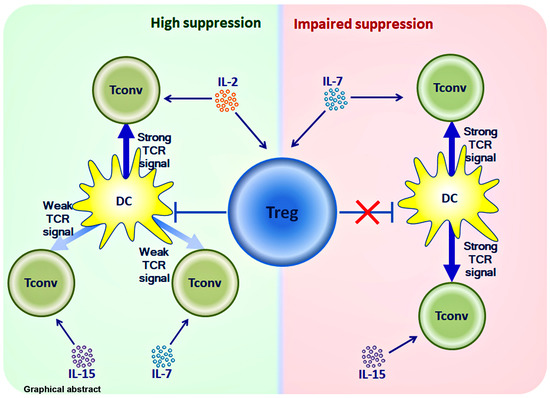
Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cell Sorting and Treg Suppressive Activity Assay
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Strong T Cell Receptor (TCR) Signal in Combination with the Influence of Homeostatic Proliferation (HP) Cytokines Reduces Suppressive Activity of Tregs
3.2. The Influence of HP Cytokines on Proliferation and Expression of Functional Molecules of Treg Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulze-Koops, H. Lymphopenia and autoimmune diseases. Arthritis Res. Ther. 2004, 6, 178–180. [Google Scholar] [CrossRef] [Green Version]
- Moxham, V.F.; Karegli, J.; Phillips, R.E.; Brown, K.L.; Tapmeier, T.T.; Hangartner, R.; Sacks, S.H.; Wong, W. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J. Immunol. 2008, 180, 3910–3918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.M.; Kaufmann, G.R.; Hodgkin, P.D.; Lewin, S.R.; Kelleher, A.D.; Davenport, M.P.; Zaunders, J.J. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol. Cell Biol. 2003, 81, 487–495. [Google Scholar] [CrossRef]
- Miller, C.N.; Hartigan-O’Connor, D.J.; Lee, M.S.; Laidlaw, G.; Cornelissen, I.P.; Matloubian, M.; Coughlin, S.R.; McDonald, D.M.; McCune, J.M. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int. Immunol. 2013, 25, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.M.; Rivas, S.C.; Colpitts, S.L.; Howard, M.E.; Stonier, S.W.; Schluns, K.S. Inflammatory Signals Regulate IL-15 in Response to Lymphodepletion. J. Immunol. 2016, 196, 4544–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Q.; Rao, V.P.; Cho, B.K.; Eisen, H.N.; Chen, J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/ MHC epitopes and strength of their interaction with T cell receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 1728–1733. [Google Scholar] [CrossRef] [Green Version]
- Yamaki, S.; Ine, S.; Kawabe, T.; Okuyama, Y.; Suzuki, N.; Soroosh, P.; Mousavi, S.F.; Nagashima, H.; Sun, S.L.; So, T.; et al. OX40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4⁺ T cells. Eur. J. Immunol. 2014, 44, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Li, O.; Zheng, P.; Liu, Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J. Exp. Med. 2004, 200, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Revell, P.A. Production and distribution of interleukin 15 and its receptors (IL-15Ralpha and IL-R2beta) in the implant interface tissues obtained during revision of failed total joint replacement. Int. J. Exp. Pathol. 2001, 82, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Regamey, N.; Obregon, C.; Ferrari-Lacraz, S.; van Leer, C.; Chanson, M.; Nicod, L.P.; Geiser, T. Airway epithelial IL-15 transforms monocytes into dendritic cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Yoon, S.O.; Armitage, R.J.; Choi, Y.S. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J. Immunol. 2004, 173, 6676–6683. [Google Scholar] [CrossRef]
- Niu, N.; Qin, X. New insights into IL-7 signaling pathways during early and late T cell development. Cell Mol. Immunol. 2013, 10, 187–189. [Google Scholar] [CrossRef]
- Mishra, A.; Sullivan, L.; Caligiuri, M.A. Molecular pathways: Interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014, 20, 2044–2050. [Google Scholar] [CrossRef] [Green Version]
- Min, B. Spontaneous T Cell Proliferation: A Physiologic Process to Create and Maintain Homeostatic Balance and Diversity of the Immune System. Front. Immunol. 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V.; Manova, O.; Kozlov, V. Homeostatic proliferation as a physiological process and a risk factor for autoimmune pathology. AIMS Allergy Immunol. 2021, 5, 18–32. [Google Scholar] [CrossRef]
- Hawse, W.F.; Cattley, R.T. T cells transduce T-cell receptor signal strength by generating different phosphatidylinositols. J. Biol. Chem. 2019, 294, 4793–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, B.; Yamane, H.; Hu-Li, J.; Paul, W.E. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 2005, 174, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Kieper, W.C.; Burghardt, J.T.; Surh, C.D. A role for TCR affinity in regulating naive T cell homeostasis. J. Immunol. 2004, 172, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theofilopoulos, A.N.; Dummer, W.; Kono, D.H. T cell homeostasis and systemic autoimmunity. J. Clin. Investig. 2001, 108, 335–340. [Google Scholar] [CrossRef]
- Jones, J.L.; Thompson, S.A.; Loh, P.; Davies, J.L.; Tuohy, O.C.; Curry, A.J.; Azzopardi, L.; Hill-Cawthorne, G.; Fahey, M.T.; Compston, A.; et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, 20200–20205. [Google Scholar] [CrossRef] [Green Version]
- Bolton, H.A.; Zhu, E.; Terry, A.M.; Guy, T.V.; Koh, W.P.; Tan, S.Y.; Power, C.A.; Bertolino, P.; Lahl, K.; Sparwasser, T.; et al. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J. Clin. Investig. 2015, 125, 3627–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, P. Functional defects of Treg Cells: New targets in rheumatic diseases, including ankylosing spondylitis. Curr. Rheumatol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, W.; Pan, W.; Zhang, Q.; Wang, G.; Feng, D.; Geng, X.; Yan, X.; Li, S. Tim3+ Foxp3 + Treg Cells are potent inhibitors of effector T Cells and are suppressed in rheumatoid arthritis. Inflammation 2017, 40, 1342–1350. [Google Scholar] [CrossRef]
- Morita, T.; Shima, Y.; Wing, J.B.; Sakaguchi, S.; Ogata, A.; Kumanogoh, A. The Proportion of Regulatory T Cells in Patients with Rheumatoid Arthritis: A Meta-Analysis. PLoS ONE 2016, 11, e0162306. [Google Scholar] [CrossRef] [Green Version]
- Flores-Borja, F.; Jury, E.C.; Mauri, C.; Ehrenstein, M.R. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2008, 105, 19396–19401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, X.; Zhuang, L.; Xu, C.; Li, T.; Zhang, G.; Liu, Y. Decreased regulatory T-cell frequency and interleukin-35 levels in patients with rheumatoid arthritis. Exp. Ther. Med. 2018, 16, 5366–5372. [Google Scholar] [CrossRef] [Green Version]
- Massalska, M.; Radzikowska, A.; Kuca-Warnawin, E.; Plebanczyk, M.; Prochorec-Sobieszek, M.; Skalska, U.; Kurowska, W.; Maldyk, P.; Kontny, E.; Gober, H.J.; et al. CD4+FOXP3+ T Cells in rheumatoid arthritis bone marrow are partially impaired. Cells 2020, 9, 549. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, B.; Chen, X.; Oppenheim, J.J.; Baeyens, A.; Gregoire, S.; Chader, D.; Gorochov, G.; Miyara, M.; Salomon, B.L. Suppressive activity of human regulatory T cells is maintained in the presence of TNF. Nat. Med. 2016, 22, 16–17. [Google Scholar] [CrossRef]
- Rossetti, M.; Spreafico, R.; Consolaro, A.; Leong, J.Y.; Chua, C.; Massa, M.; Saidin, S.; Magni-Manzoni, S.; Arkachaisri, T.; Wallace, C.A.; et al. TCR repertoire sequencing identifies synovial Treg cell clonotypes in the bloodstream during active inflammation in human arthritis. Ann. Rheum. Dis. 2017, 76, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Han, G.M.; O’Neil-Andersen, N.J.; Zurier, R.B.; Lawrence, D.A. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008, 253, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, G.J.; Fleskens, V.; Frederiksen, K.S.; Rajasekhar, M.; Menon, B.; Gerwien, J.G.; Evans, H.G.; Taams, L.S. Phenotypic, functional, and gene expression profiling of peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127(low) Treg Cells in patients with chronic rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Wang, W.; Jia, R.; Li, J.; You, H.; Chen, L.; Wang, Y. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand. J. Rheumatol. 2007, 36, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Vignali, D.A. In vitro Treg suppression assays. Methods Mol. Biol. 2011, 707, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Wang, S.; Miao, R.; Kan, W. TRAIL is associated with impaired regulation of CD4+CD25- T cells by regulatory T cells in patients with rheumatoid arthritis. J. Clin. Immunol. 2011, 31, 1112–1119. [Google Scholar] [CrossRef]
- Ashley, C.W.; Baecher-Allan, C. Cutting Edge: Responder T cells regulate human DR+ effector regulatory T cell activity via granzyme B. J. Immunol. 2009, 183, 4843–4847. [Google Scholar] [CrossRef] [Green Version]
- Zembruski, N.C.; Stache, V.; Haefeli, W.E.; Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 2012, 429, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Basdeo, S.A.; Moran, B.; Cluxton, D.; Canavan, M.; McCormick, J.; Connolly, M.; Orr, C.; Mills, K.H.; Veale, D.J.; Fearon, U.; et al. Polyfunctional, Pathogenic CD161+ Th17 Lineage Cells Are Resistant to Regulatory T Cell-Mediated Suppression in the Context of Autoimmunity. J. Immunol. 2015, 195, 528–540. [Google Scholar] [CrossRef] [Green Version]
- Smigielska-Czepiel, K.; van den Berg, A.; Jellema, P.; van der Lei, R.J.; Bijzet, J.; Kluiver, J.; Boots, A.M.; Brouwer, E.; Kroesen, B.J. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory Tregs. Genes Immun. 2014, 15, 115–125. [Google Scholar] [CrossRef]
- Wehrens, E.J.; Mijnheer, G.; Duurland, C.L.; Klein, M.; Meerding, J.; van Loosdregt, J.; de Jager, W.; Sawitzki, B.; Coffer, P.J.; Vastert, B.; et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood 2011, 118, 3538–3548. [Google Scholar] [CrossRef] [Green Version]
- Ernestam, S.; af Klint, E.; Catrina, A.I.; Sundberg, E.; Engström, M.; Klareskog, L.; Ulfgren, A.K. Synovial expression of IL-15 in rheumatoid arthritis is not influenced by blockade of tumour necrosis factor. Arthritis Res. Ther. 2006, 8, R18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Roon, J.A.; Verweij, M.C.; Wijk, M.W.; Jacobs, K.M.; Bijlsma, J.W.; Lafeber, F.P. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005, 52, 1700–1710. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, Q.T.; Lee, J.; Lee, S.H.; Janocha, A.; Kim, S.; Le, H.T.; Dvorina, N.; Weiss, K.; Cameron, M.J.; et al. Anti-inflammatory roles of glucocorticoids are mediated by Foxp3+ Regulatory T Cells via a miR-342-dependent mechanism. Immunity 2020, 53, 581–596.e5. [Google Scholar] [CrossRef]
- Kassiotis, G.; Zamoyska, R.; Stockinger, B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J. Exp. Med. 2003, 197, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Heninger, A.K.; Theil, A.; Wilhelm, C.; Petzold, C.; Huebel, N.; Kretschmer, K.; Bonifacio, E.; Monti, P. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J. Immunol. 2012, 189, 5649–5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchman, S.M.; El-Jawhari, J.J.; Burska, A.N.; Parmar, R.; Goëb, V.; Conaghan, P.G.; Emery, P.; Ponchel, F. Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020, 10, 3100. [Google Scholar] [CrossRef] [Green Version]
- Gianchecchi, E.; Fierabracci, A. Inhibitory receptors and pathways of lymphocytes: The role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 2018, 9, 2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajram, L.; Begg, M.; Slack, R.; Cryan, J.; Hall, D.; Hodgson, S.; Ford, A.; Barnes, A.; Swieboda, D.; Mousnier, A.; et al. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur. J. Pharmacol. 2014, 729, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Bromley, S.K.; Means, T.K.; Jones, K.J.; Hayashi, F.; Bhan, A.K.; Luster, A.D. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J. Exp. Med. 2007, 204, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950. [Google Scholar] [CrossRef] [Green Version]
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V.; Sizikov, A.; Chumasova, O.; Koksharova, V. T-regulatory cells from patients with rheumatoid arthritis retain suppressor functions in vitro. Exp. Ther. Med. 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.V.; Blinova, E.A.; Kozlov, V.A. The influence of humoral factors of homeostatic proliferation on t-regulatory cells in vitro. Bull. Sib. Med. 2019, 18, 286–293. [Google Scholar] [CrossRef]
- Peiser, M.; Becht, A.; Wanner, R. Antibody blocking of MHC II on human activated regulatory T cells abrogates their suppressive potential. Allergy 2007, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’Shea, M.A.; Fauci, A.S. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 2008, 181, 6738–6746. [Google Scholar] [CrossRef]










| Parameter | Healthy Donors | RA Patients |
|---|---|---|
| Male | 6 | 3 |
| Female | 12 | 11 |
| Age, mean ± SD | 56.4 ± 11 | 59.5 ± 11 |
| DAS-28, mean ± SD | - | 5.2 ± 1.6 |
| ESR (mm/h), mean ± SD | - | 35.4 ± 9.4 |
| CRP (mg/dl), mean ± SD | - | 19.9 ± 6.7 |
| TJC, mean ± SD, out of 28 | - | 7.3 ± 3.2 |
| SJC, mean ± SD, out of 28 | - | 4.2 ± 2.9 |
| Duration of disease (y) | - | 5.5 ± 2.7 |
| Groups | N | Age (Mean ± SD) | Male | Female | Duration (Mean ± SD) | DAS-28 (Mean ± SD) |
|---|---|---|---|---|---|---|
| DAS-28 < 3.2 | 4 | 62.5 ± 18.9 | - | 4 | 15 ± 2.1 years | 2.01 ± 0.82 |
| DAS-28 > 3.2 | 9 | 59.1 ± 9.1 | 1 | 8 | 6 ± 4.6 years | 5.58 ± 0.99 |
| RA < 1 year | 4 | 60 ± 4.5 | - | 4 | 9 ± 2 months | 5.92 ± 1.61 |
| RA > 1 year | 9 | 59.7 ± 13.2 | 1 | 8 | 10.1 ± 4.8 years | 4.10 ± 1.78 |
| HD | 18 | 56.4 ± 11 | 6 | 12 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevyrev, D.; Tereshchenko, V.; Blinova, E.; Knauer, N.; Pashkina, E.; Sizikov, A.; Kozlov, V. Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro. Life 2021, 11, 245. https://doi.org/10.3390/life11030245
Shevyrev D, Tereshchenko V, Blinova E, Knauer N, Pashkina E, Sizikov A, Kozlov V. Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro. Life. 2021; 11(3):245. https://doi.org/10.3390/life11030245
Chicago/Turabian StyleShevyrev, Daniil, Valeriy Tereshchenko, Elena Blinova, Nadezda Knauer, Ekaterina Pashkina, Alexey Sizikov, and Vladimir Kozlov. 2021. "Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro" Life 11, no. 3: 245. https://doi.org/10.3390/life11030245
APA StyleShevyrev, D., Tereshchenko, V., Blinova, E., Knauer, N., Pashkina, E., Sizikov, A., & Kozlov, V. (2021). Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro. Life, 11(3), 245. https://doi.org/10.3390/life11030245