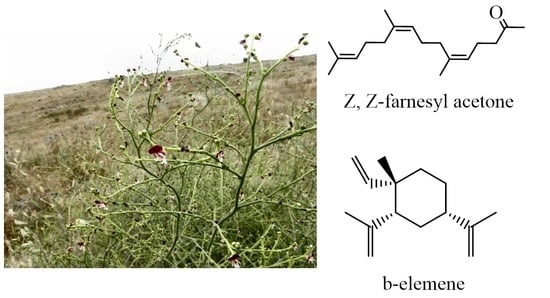
Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemical Reagents
2.3. Plant Material and Fractionations
2.4. Extraction of Essential Oils
2.5. Phytochemical Analysis
2.6. GC/MS Analysis of Essential Oils
Identification of the Chemical Constituents
2.7. Determination of Antioxidant Activity
2.7.1. DPPH Free Radical Scavenging Activity
2.7.2. ABTS Radical Scavenging Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of S. peyronii EO
3.2. Phytochemical Analysis
3.3. Phytochemical Profiling of Crude Extracts by Using LC-MS/MS
| No. | Rt (min) | m/z Meas. | MM Calculated | Name | Structure | Molecular Formula | Fractions | Classification | |
|---|---|---|---|---|---|---|---|---|---|
| Sp-M | Sp-B | ||||||||
| 1 | 0.99 | 117.0176 | 118.049 | Succinic acid |  | C4H6O4 | + | + | Aliphatic acid |
| 2 | 2.14 | 375.1257 | 376.133 | 3,4-Dihydro-methyl catalpol |  | C16H24O10 | + | + | Iridoid |
| 3 | 3.45 | 625.1359 | 626.1432 | Isorhamnetin-3-O-rutinoside |  | C28H32O16 | + | + | Flavonoid |
| 4 | 3.81 | 811.2823 | 812.2896 | Scropolioside B |  | C41H46O17 | − | + | Iridoid |
| 5 | 4.15 | 179.0325 | 180.0398 | Caffeic acid |  | C9H8O4 | + | + | Phenolic acid |
| 6 | 4.49 | 163.0379 | 164.0452 | p-Coumaric acid |  | C9H8O3 | + | + | Phenolic acid |
| 7 | 5.41 | 609.1411 | 610.1484 | Rutin |  | C27H30O16 | + | + | Flavonoid |
| 8 | 5.61 | 609.1395 | 610.1467 | 3-Glu-7-Rha Quercetin |  | C27H30O16 | + | + | Flavonoid |
| 9 | 5.76 | 495.203 | 496.2103 | 6′-O-cinnamoylharpagide |  | C24H30O11 | − | + | Iridoid |
| 10 | 5.78 | 175.0377 | 194.0554 | Isoferulic acid |  | C10H10O4 | − | + | Phenolic acid |
| 11 | 5.78 | 463.0837 | 464.0909 | Hyperoside |  | C21H20O12 | + | + | Flavonoid |
| 12 | 6.35 | 593.1463 | 594.1535 | 3-O-Neohesperidoside Kaempferol |  | C27H30O15 | + | + | Flavonoid |
| 13 | 6.56 | 447.0892 | 448.0965 | Kaempferol-3-O-glucoside |  | C21H20O11 | + | + | Flavonoid |
| 14 | 6.57 | 495.3129 | 496.3202 | Nepitrin |  | C22H22O13 | + | + | Flavonoid |
| 15 | 7.96 | 377.0807 | 756.1759 | 6-O-Methylcatapol |  | C16H24O10 | − | + | Iridoid |
| 16 | 8.72 | 147.0427 | 148.05 | Cinnamic acid |  | C9H8O2 | + | + | Phenolic acid |
| 17 | 11.09 | 751.2377 | 752.2449 | Scropolioside A |  | C35H44O18 | + | + | Iridoid |
| 18 | 13.67 | 752.2377 | 753.245 | Scrovalentinoside |  | C35H44O18 | + | + | Iridoid |
| 19 | 27.05 | 478.3855 | 479.3928 | Homoplantaginin |  | C22H22O11 | + | + | Flavonoid |
| 20 | 29.97 | 351.2467 | 352.254 | Ningposide C |  | C17H20O8 | + | + | Phenolic acid |
| 21 | 30.07 | 339.3222 | 340.3295 | 3-O-trans-Feruloylrhamnopyranose |  | C16H20O8 | + | + | Phenolic acid |
3.4. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandyopadhyay, M.; Chakraborty, R.; Raychaudhuri, U. A process for preparing a natural antioxidant enriched dairy product (Sandesh). LWT Food Sci. Technol. 2007, 40, 842–851. [Google Scholar] [CrossRef]
- Zohary, M.; Feinbrun-Dothan, N. Flora Palastina; Israel Academy of Sciences and Humanitie: Jerusalem, Israel, 1966. [Google Scholar]
- Oran, S.A.S. Flora of Bader Al-Jadida County, western high mountains of Amman city/Jordan. Int. J. Herb. Med. 2015, 3, 49–59. [Google Scholar]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giner, R.M.; Villalba, M.L.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Rίos, J.L. Anti-inflammatory glycoterpenoids from Scrophularia auriculata. Eur. J. Pharmacol. 2000, 389, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Gross, G.A.; Sticher, O. Two phenylpropanoid glycosides from Scrophularia scopolii. Phytochemistry 1988, 27, 1465–1468. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Fontaine, J.; Malonne, H.; Claeys, M.; Luhmer, M.; Duez, P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry 2005, 66, 1186–1191. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Hajiaghaee, R.; Shahverdi, A.R.; Khorramizadeh, M.R.; Amini, M. Flavonoids, cinnamic acid and phenyl propanoid from aerial parts of Scrophularia striata. Pharm. Biol. 2010, 48, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.P.S.; Babu, U.V.; Garg, H.S. A triterpene glycoside from Scrophularia koelzii. Phytochemistry 1997, 45, 1717–1719. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Simmonds, M.S.; Sampson, J.; Houghton, P.J.; Grice, P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother. Res. 2002, 16, 33–35. [Google Scholar] [CrossRef]
- Mahmoud, B.; Hadavi, M.; Abbasi, N. Study of extraction and chemical compounds of Scrophularia striata Boiss. and Scrophularia deserti Delile using HS-SPME and GC-MS. Plant Biotechnol. Persa 2020, 2, 8–13. [Google Scholar] [CrossRef]
- Dıaz, A.M.; Abad, M.J.; Fernández, L.; Silván, A.M.; De Santos, J.; Bermejo, P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004, 74, 2515–2526. [Google Scholar] [CrossRef]
- Panda, P.; Appalashetti, M.; MA Judeh, Z. Phenylpropanoid sucrose esters: Plant-derived natural products as potential leads for new therapeutics. Curr. Med. Chem. 2011, 18, 3234–3251. [Google Scholar] [CrossRef]
- Li, Y.M.; Jiang, S.H.; Gao, W.Y.; Zhu, D.Y. Phenylpropanoid glycosides from Scrophularia ningpoensis. Phytochemistry 2000, 54, 923–925. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Saenz, M.T.; Garcia, M.D. Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 1998, 50, 1183–1186. [Google Scholar] [CrossRef]
- Zhu, L.J.; Hou, Y.L.; Shen, X.Y.; Pan, X.D.; Zhang, X.; Yao, X.S. Monoterpene pyridine alkaloids and phenolics from Scrophularia ningpoensis and their cardioprotective effect. Fitoterapia 2013, 88, 44–49. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, Y.C. Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana. Phytochemistry 2000, 54, 503–509. [Google Scholar] [CrossRef]
- Baradaran, P.C.; Mohammadi, A.; Mansoori, B.; Baradaran, S.C.; Baradaran, B. Growth inhibitory effect of Scrophularia oxysepala extract on mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Biomed. Pharmacother. 2017, 85, 718–724. [Google Scholar] [CrossRef]
- Alqasoumi, S.I. Evaluation of the hepatroprotective and nephroprotective activities of Scrophularia hypericifolia growing in Saudi Arabia. Saudi Pharm. J. 2014, 22, 258–263. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, K.Y.; Koo, K.A.; Sung, S.H.; Lee, N.G.; Kim, J.; Kim, Y.C. Four new neuroprotective iridoid glycosides from Scrophularia buergeriana roots. J. Nat. Prod. 2002, 65, 1696–1699. [Google Scholar] [CrossRef]
- Sohn, S.H.; Ko, E.; Jeon, S.B.; Lee, B.J.; Kim, S.H.; Dong, M.S.; Lee, D.; Kwak, J.; Kim, Y.; Shin, M.; et al. The genome-wide expression profile of Scrophularia ningpoensis-treated thapsigargin-stimulated U-87MG cells. Neurotoxicology 2009, 30, 368–376. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Jaber, H.I.; Zarga, M.H.A.; Orabi, S.T.A. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidi, J.Y.; Al-Qudah, M.A.; Al-Saleem, M.S.; Alotaibi, S.M. Antioxidant activity and chemical composition of essential oils of selected Cleome species growing in Saudi Arabia. Jordan J. Chem. 2019, 14, 29–37. Available online: https://jjc.yu.edu.jo/index.php/jjc/article/view/25 (accessed on 1 May 2023).
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Al-Jaber, H.I.; Tashtoush, H.I.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.F.; Al-Sheraideh, M.S.; Mayyas, A.S.; Abu-Orabi, S.T.; Al-Qudah, M.A. LC-MS/MS screening, total phenolic, flavonoid and antioxidant contents of crude extracts from three Asclepiadaceae species growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.Q.; Almasarweh, A.B.; Abdo, N.M.; Mayyas, A.S.; Al-Qudah, M.A.; Abu-Orabi, S.T. Chromatographic analysis (LC-MS and GC-MS), antioxidant activity, antibacterial activity, total phenol, and total flavonoid determination of Cleome arabica L. growing in Jordan. Int. J. Food Prop. 2022, 25, 1920–1933. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Migdadi, R.S.; Mayyas, A.S.; Al-Zereini, W.A.; Al-Dalahmeh, Y.; Abu Orabi, F.M.; Bataineh, T.T.; Abu-Orabi, S.T. Chemical Composition, Cytotoxicity and Antioxidant Activity of the Essential Oil from Flower Buds and Leaves of the Pulicaria incisa (Lam.) DC and Pulicaria crispa (Forskel) Oliver. J. Essent. Oil-Bear. Plants 2022, 25, 758–772. [Google Scholar] [CrossRef]
- Lo’ay, A.; Abu-Orabi, S.T.; Hlail, H.M.; Alkhatib, R.Q.; Al-Dalahmeh, Y.; Al-Qudah, M.A. Anthemis cotula L. from Jordan: Essential oil composition, LC-ESI-MS/MS profiling of phenolic acids-flavonoids and in vitro antioxidant activity. Arab. J. Chem. 2023, 16, 104470. [Google Scholar] [CrossRef]
- Al-Bataineh, N.; Algethami, F.K.; Al-Jaber, H.I.; Alhamzani, A.G.; Bataineh, R.M.; Al-Dalahmeh, Y.; Bataineh, T.T.; Abu-Orabi, S.T.; Al-Qudah, M.A. Ballota saxatilis from Jordan: Evaluation of Essential Oil Composition and Phytochemical Profiling of Crude Extracts and Their In-Vitro Antioxidant Activity. Separations 2023, 10, 114. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Smadi, Z.M.; Al-Jaber, H.I.; Tashtoush, H.I.; Alkhatib, R.Q.; Bataineh, T.T.; Al-Dalahmeh, Y.; Orabi, S.T.A. GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity. Arab. J. Chem. 2023, 16, 104641. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Ali, M. Practical Pharmaceutical Chemistry; CBS Publishers & Distributors: New Delhi, India, 1997. [Google Scholar]
- Wadood, A.; Ghufran, M.; Jamal, S.B.; Naeem, M.; Khan, A.; Ghaffar, R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem. Anal Biochem. 2013, 2, 144. [Google Scholar] [CrossRef]
- Banu, K.S.; Cathrine, L. General techniques involved in phytochemical analysis. Int. J. Adv. Res. Chem. Sci. 2015, 2, 25–32. [Google Scholar]
- Ajayi, I.A.; Ajibade, O.; Oderinde, R.A. Preliminary phytochemical analysis of some plant seeds. Res. J. Chem. Sci. 2011, 1, 58–62. [Google Scholar]
- Pasdaran, A.; Nahar, L.; Asnaashari, S.; Sarker, S.D.; Delazar, A. Gc-ms analysis, free-radical-scavenging and insecticidal activities of essential oil of Scrophularia oxysepala boiss. Pharm. Sci. 2013, 19, 1–5. [Google Scholar]
- Miyazawa, M.; Okuno, Y. Volatile components from the roots of Scrophularia ningpoensis Hemsl. Flavour Fragr. J. 2003, 18, 398–400. [Google Scholar] [CrossRef]
- Asgharian, P.; Afshar, F.H.; Asnaashari, S.; Moghaddam, S.B.; Delazar, A. The seasonal variations of the chemical composition of essential oil obtained from Scrophularia frigida. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Pasdaran, A.; Delazar, A.; Nazemiyeh, H.; Nahar, L.; Sarker, S.D. Chemical composition, and antibacterial (against Staphylococcus aureus) and free-radical-scavenging activities of the essential oil of Scrophularia amplexicaulis Benth. Rec. Nat. Prod. 2012, 6, 350–355. [Google Scholar]
- Asgharian, P.; Afshar, F.H.; Asnaashari, S.; Moghaddam, S.B.; Ebrahimi, A.; Delazar, A. Characterization of terpenoids in the essential oil extracted from the aerial parts of Scrophularia subaphylla growing in Iran. Adv. Pharm. Bull. 2015, 5, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Mardani, M.; Rezapour, S.; Eftekhari, Z.; Majid, A.S.; Rashidipour, M.; Afsordeh, O.; Kazemzadeh, F.; Bahmani, F. Chemical composition of Scrophularia deserti. Int. J. Pharm. Tech Res. 2016, 9, 285–290. [Google Scholar] [CrossRef]
- Nikkhah, E.; Asnaashari, S.; Babaei, H.; Heshmati Afshar, F.; Delazar, A. Chemical composition and biological activities of essential oil and methanol extract of Scrophularia umbrosa. Res. J. Pharmacogn. 2017, 4, 41–50. [Google Scholar]
- Kerdar, T.; Moradkhani, S.; Dastan, D. Phytochemical and biological studies of Scrophularia striata from Ilam. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e62705. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.Y.; Qin, L.P.; Zhu, B. Pharmacology, phytochemistry, and traditional uses of Scrophularia ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.A.; Kim, H.L.; Lee, D.R.; Choi, B.K.; Yang, S.H. Anti-inflammatory effects of Scrophularia buergeriana extract mixture fermented with lactic acid bacteria. Biotechnol. Bioprocess Eng. 2022, 27, 370–378. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G.; Zhao, Z.; Li, Y. Iridoid glycosides isolated from Scrophularia dentata Royle ex Benth. and their anti-inflammatory activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lympho-cytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphe-nols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Formagio, A.S.N. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guin-eense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]



| No. | Rt (min) | RI (exp) | Compound | % Peak Area | Method of Identification |
|---|---|---|---|---|---|
| 1 | 3.676 | 877 | (4Z)-Hexenol | 0.56 | MS, RI |
| 2 | 5.847 | 954 | (2E)-Heptenal | 0.47 | MS, RI |
| 3 | 5.955 | 960 | Benzaldehyde | 0.94 | MS, RI |
| 4 | 6.48 | 998 | n-Octanal | 5.98 | MS, RI |
| 5 | 6.698 | 1004 | ρ-Mentha-1(7),8-diene | 0.47 | MS, RI |
| 6 | 6.819 | 1007 | (2E,4E)-Heptadienal | 0.99 | MS, RI, RC |
| 7 | 7.168 | 1013 | (2E)-Hexenyl acetate | 0.90 | MS, RI |
| 8 | 8.035 | 1029 | Limonene | 4.10 | MS, RI |
| 9 | 8.431 | 1039 | Lavender lactone | 0.55 | MS, RI, RC |
| 10 | 8.522 | 1042 | Benzene acetaldehyde | 0.52 | MS, RI, RC |
| 11 | 9.086 | 1059 | trans-Decahydro-naphthalene | 0.77 | MS, RI |
| 12 | 9.364 | 1065 | Acetophenone | 0.52 | MS, RI |
| 13 | 9.579 | 1081 | cis-Vertocitral | 0.48 | MS, RI |
| 14 | 9.598 | 1088 | Camphenilone | 0.81 | MS, RI |
| 15 | 10.184 | 1096 | Linalool | 0.49 | MS, RI |
| 16 | 10.46 | 1103 | 2,2-Dimethyl-3,4-Octadienal | 0.62 | MS, RI |
| 17 | 10.662 | 1120 | Dehydro-Sabina ketone | 3.76 | MS, RI |
| 18 | 10.796 | 1125 | 1-Undecyne | 2.24 | MS, RI, RC |
| 19 | 10.917 | 1126 | α-Campholenal | 1.02 | MS, RI |
| 20 | 12.526 | 1146 | Menthone | 0.48 | MS, RI |
| 21 | 13.073 | 1162 | iso-Menthone | 0.68 | MS, RI |
| 22 | 13.336 | 1169 | Borneol | 0.89 | MS, RI |
| 23 | 13.488 | 1177 | Santalone | 2.02 | MS, RI |
| 24 | 13.652 | 1177 | cis-Pinocarveol | 0.48 | MS, RI |
| 25 | 13.801 | 1189 | trans-ρ-Mentha-1(7),8-dien-2-ol | 0.86 | MS, RI |
| 26 | 14.392 | 1199 | γ-Terpineol | 1.57 | MS, RI |
| 27 | 14.699 | 1217 | 4-Methylene-Isophorone | 0.62 | MS, RI |
| 28 | 14.982 | 1225 | Citronellol | 0.92 | MS, RI |
| 29 | 15.589 | 1237 | Pulegone | 1.06 | MS, RI |
| 30 | 17.386 | 1263 | (2E)-Decenal | 1.47 | MS, RI |
| 31 | 17.502 | 1269 | n-Decanol | 0.54 | MS, RI |
| 32 | 18.007 | 1286 | 5-Undecanol | 0.49 | MS, RI |
| 33 | 18.407 | 1294 | Camphorquinone | 0.71 | MS, RI |
| 34 | 18.914 | 1305 | iso-Menthyl acetate | 0.86 | MS, RI, RC |
| 35 | 19.597 | 1314 | 2,3,4-Trimethyl benzaldehyde | 1.67 | MS, RI |
| 36 | 19.747 | 1324 | trans-(E)-Jasmonol | 1.11 | MS, RI |
| 37 | 20.824 | 1352 | Citronellyl acetate | 2.07 | MS, RI |
| 38 | 21.755 | 1375 | Linalool isobutanoate | 1.29 | MS, RI |
| 39 | 22.565 | 1393 | β-Elemene | 6.36 | MS, RI |
| 40 | 22.861 | 1407 | Longifolene | 4.30 | MS, RI |
| 41 | 23.487 | 1408 | Dodecanal | 0.69 | MS, RI |
| 42 | 24.003 | 1433 | β-Gurjunene | 4.16 | MS, RI, RC |
| 43 | 24.549 | 1439 | α-Guaiene | 0.48 | MS, RI |
| 44 | 25.428 | 1459 | trans-Prenyl limonene | 0.99 | MS, RI |
| 45 | 25.606 | 1460 | allo-Aromadendrene | 0.53 | MS, RI |
| 46 | 25.869 | 1466 | (2E)-Dodecenal | 1.31 | MS, RI |
| 47 | 26.764 | 1488 | (E)-β-Ionone | 2.06 | MS, RI |
| 48 | 27.158 | 1502 | trans-β-Guaiene | 0.61 | MS, RI |
| 49 | 27.714 | 1513 | γ-Cadinene | 1.84 | MS, RI |
| 50 | 28.269 | 1534 | trans-Cadina-1,4-diene | 0.50 | MS, RI |
| 51 | 29.9 | 1578 | Spathulenol | 4.58 | MS, RI |
| 52 | 30.089 | 1580 | n-Hexyl benzoate | 0.54 | MS, RI |
| 53 | 30.368 | 1590 | β-Copaen-4-α-ol | 0.72 | MS, RI |
| 54 | 30.549 | 1604 | Khusimone | 1.52 | MS, RI |
| 55 | 31.757 | 1623 | 10-epi-γ-Eudesmol | 0.60 | MS, RI |
| 56 | 32.154 | 1641 | allo-Aromadendrene epoxide | 1.25 | MS, RI |
| 57 | 32.815 | 1646 | α-Muurolol | 3.15 | MS, RI |
| 58 | 33.313 | 1660 | neo-Intermedeol | 0.66 | MS, RI |
| 59 | 34.06 | 1679 | (Z)-Methyl epi-jasmonate | 1.07 | MS, RI, |
| 60 | 34.349 | 1688 | Eudesma-4(15),7-dien-1β-ol | 0.49 | MS, RI |
| 61 | 34.503 | 1699 | 11-αH-Himachal-4-en-1-β-ol | 0.48 | MS, RI |
| 62 | 38.085 | 1792 | Drimenone | 0.54 | MS, RI |
| 63 | 40.143 | 1856 | Z,Z-Farnesyl acetone | 11.04 | MS, RI |
| 64 | 41.642 | 1913 | (5E,9E)-Farnesyl acetone | 0.60 | MS, RI |
| 65 | 42.907 | 1942 | Callitrisin | 0.58 | MS, RI |
| 66 | 43.95 | 1971 | (Z)-Methyl-isoprenyl cinnamate | 0.53 | MS, RI |
| Total identified | 98.16 |
| Groups | Sp-M | Sp-B |
|---|---|---|
| Flavonoids | + | + |
| Glycosides | + | + |
| Alkaloids | + | − |
| Tannins | − | − |
| Saponins | + | + |
| Anthraquinone | + | + |
| Extract | TPC | TFC | IC50 (mg/mL) | |
|---|---|---|---|---|
| DPPH• | ABTS | |||
| Sp-M | 45.44 ± 1.3 * | 25.74 ± 2.4 | (8.6 ± 0.09) × 10−2 | (26.0 ± 0.02) × 10−2 |
| Sp-B | 51.90 ± 1.0 | 148.54 ± 1.0 | (8.0 ± 0.08) × 10−2 | (2.5 ± 0.02) × 10−2 |
| EO | - | - | (4.84 ± 0.12) × 10−3 | (10.6 ± 0.08) × 10−3 |
| Ascorbic acid | - | - | (1.8 ± 0.06) × 10−3 | (1.9 ± 0.04 ) × 10−3 |
| α-tocopherol | - | - | (2.3 ± 0.04) × 10−3 | (1.8 ± 0.01) × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dalahmeh, Y.; Almahmoud, S.A.J.; Al-Bataineh, N.; Alghzawi, T.A.; Alhamzani, A.G.; Al-Mutairi, A.A.; Al-Jaber, H.I.; Abu Orabi, S.T.; Bataineh, T.T.; Al-Sheraideh, M.S.; et al. Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity. Life 2023, 13, 1404. https://doi.org/10.3390/life13061404
Al-Dalahmeh Y, Almahmoud SAJ, Al-Bataineh N, Alghzawi TA, Alhamzani AG, Al-Mutairi AA, Al-Jaber HI, Abu Orabi ST, Bataineh TT, Al-Sheraideh MS, et al. Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity. Life. 2023; 13(6):1404. https://doi.org/10.3390/life13061404
Chicago/Turabian StyleAl-Dalahmeh, Yousef, Sondos Abdullah J. Almahmoud, Nezar Al-Bataineh, Taqwa A. Alghzawi, Abdulrahman G. Alhamzani, Aamal A. Al-Mutairi, Hala I. Al-Jaber, Sultan T. Abu Orabi, Tareq T. Bataineh, Mohammed S. Al-Sheraideh, and et al. 2023. "Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity" Life 13, no. 6: 1404. https://doi.org/10.3390/life13061404
APA StyleAl-Dalahmeh, Y., Almahmoud, S. A. J., Al-Bataineh, N., Alghzawi, T. A., Alhamzani, A. G., Al-Mutairi, A. A., Al-Jaber, H. I., Abu Orabi, S. T., Bataineh, T. T., Al-Sheraideh, M. S., & Al-Qudah, M. A. (2023). Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity. Life, 13(6), 1404. https://doi.org/10.3390/life13061404