Limited Sequence Diversity Within a Population Supports Prebiotic RNA Reproduction
Abstract
:1. Introduction
2. Materials and Methods
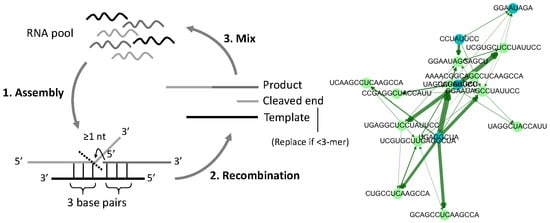
2.1. Model Description
2.2. Graph Analysis of Simulation
3. Results
3.1. Directed Graph Representations
3.2. The Highest Chance of Reproduction is at Intermediate Sequence Diversity within A Population
3.3. There Are Too Many Unrelated Recombination Reactions at High Sequence Diversity within a Population
3.4. The Effect of Population Size on the Range of Intermediate Diversity
3.5. Comparison of Template-Directed Recombination with Template-Directed Ligation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Sutherland, J.D. Opinion: Studies on the origin of life-The end of the beginning. Nat. Rev. Chem. 2017, 1, 0012. [Google Scholar] [CrossRef]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Gilbert, W. The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Joyce, G.F. RNA evolution and the origins of life. Nature 1989, 338, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hill, A.R.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Prywes, N.; Blain, J.C.; Del Frate, F.; Szostak, J.W. Nonenzymatic copying of RNA templates containing all four letters is catalyzed by activated oligonucleotides. Elife 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Biscans, A. Exploring the emergence of RNA nucleosides and nucleotides on the early Earth. Life 2018, 8, 57. [Google Scholar] [CrossRef]
- Derr, J.; Manapat, M.L.; Rajamani, S.; Leu, K.; Xulvi-Brunet, R.; Joseph, I.; Nowak, M.A.; Chen, I.A. Prebiotically plausible mechanisms increase compositional diversity of nucleic acid sequences. Nucleic Acids Res. 2012, 40, 4711–4722. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Orgel, L.E. A nonenzyamatic RNA polymerase model. Science 1983, 219, 859–862. [Google Scholar] [CrossRef]
- Monnard, P.A.; Kanavarioti, A.; Deamer, D.W. Eutectic phase polymerization of activated ribonucleotide mixtures yields quasi-equimolar incorporation of purine and pyrimidine nucleobases. J. Am. Chem. Soc. 2003, 125, 13734–13740. [Google Scholar] [CrossRef] [PubMed]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, S.; Kun, Á.; Ryckelynck, M.; Coldren, F.; Szilágyi, A.; Jossinet, F.; Rick, C.; Nghe, P.; Szathmáry, E.; Griffiths, A.D. Transient compartmentalization of RNA replicators prevents extinction due to parasites. Science 2016, 354, 1293–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuuchi, R.; Ichihashi, N. Sustainable replication and coevolution of cooperative RNAs in an artificial cell-like system. Nat. Ecol. Evol. 2018, 2, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Lutay, A.V.; Zenkova, M.A.; Vlassov, V.V. Nonenzymatic recombination of RNA: Possible mechanism for the formation of novel sequences. Chem. Biodivers. 2007, 4, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Lehman, N. A recombination-based model for the origin and early evolution of genetic information. Chem. Biodivers. 2008, 5, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, H.; Taylor, A.I.; Lightowler, A.; Houlihan, G.; Abramov, M.; Herdewijn, P.; Holliger, P. Innate potential of random genetic oligomer pools for recombination. Elife 2018, 7, e43022. [Google Scholar] [CrossRef] [PubMed]
- Smail, B.A.; Clifton, B.E.; Mizuuchi, R.; Lehman, N. Spontaneous advent of genetic diversity in RNA populations through multiple recombination mechanisms. RNA 2019, in press. [Google Scholar] [CrossRef]
- Sievers, D.; von Kiedrowski, G. Self-replication of complementary nucleotide-based oligomers. Nature 1994, 369, 221–224. [Google Scholar] [CrossRef]
- Rohatgi, R.; Bartel, D.P.; Szostak, J.W. Nonenzymatic, template-directed ligation of oligoribonucleotides is highly regioselective for the formation of 3′-5′ phosphodiester bonds. J. Am. Chem. Soc. 1996, 118, 3340–3344. [Google Scholar] [CrossRef]
- Nghe, P.; Hordijk, W.; Kauffman, S.A.; Walker, S.I.; Schmidt, F.J.; Kemble, H.; Yeates, J.A.M.; Lehman, N. Prebiotic network evolution: six key parameters. Mol. Biosyst. 2015, 11, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Dyson, F.J. A model for the origin of life. J. Mol. Evol. 1982, 18, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S.A. Autoeatalytic Sets of Proteins. J. Theor. Biol. 1986, 119, 1–24. [Google Scholar] [CrossRef]
- Jain, S.; Krishna, S. A model for the emergence of cooperation, interdependence and structure in evolving networks. Proc. Natl. Acad. Sci. USA 2000, 98, 543–547. [Google Scholar] [CrossRef]
- Hordijk, W.; Steel, M. Detecting autocatalytic, self-sustaining sets in chemical reaction systems. J. Theor. Biol. 2004, 227, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordijk, W.; Kauffman, S.A.; Steel, M. Required levels of catalysis for emergence of autocatalytic sets in models of chemical reaction systems. Int. J. Mol. Sci. 2011, 12, 3085–3101. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.V.; Maslov, S. Spontaneous emergence of autocatalytic information-coding polymers. J. Chem. Phys. 2015, 143, 045102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoln, T.A.; Joyce, G.F. Self-sustained replication of an RNA enzyme. Science 2009, 323, 1229–1232. [Google Scholar] [CrossRef]
- Vaidya, N.; Manapat, M.L.; Chen, I.A.; Xulvi-Brunet, R.; Hayden, E.J.; Lehman, N. Spontaneous network formation among cooperative RNA replicators. Nature 2012, 491, 72–77. [Google Scholar] [CrossRef]
- Mariani, A.; Bonfio, C.; Johnson, C.M.; Sutherland, J.D. pH-Driven RNA strand separation under prebiotically plausible conditions. Biochemistry 2018, 57, 6382–6386. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. A principle of natural self-organization—Part A: Emergence of the hypercycle. Naturwissenschaften 1977, 64, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, N.; Walker, S.I.; Lehman, N. Recycling of informational units leads to selection of replicators in a prebiotic soup. Chem. Biol. 2013, 20, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Arsene, S.; Ameta, S.; Lehman, N.; Griffiths, A.D.; Nghe, P. Coupled catabolism and anabolism in autocatalytic RNA sets. Nucleic Acids Res. 2018, 46, 9660–9666. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuuchi, R.; Lehman, N. Limited Sequence Diversity Within a Population Supports Prebiotic RNA Reproduction. Life 2019, 9, 20. https://doi.org/10.3390/life9010020
Mizuuchi R, Lehman N. Limited Sequence Diversity Within a Population Supports Prebiotic RNA Reproduction. Life. 2019; 9(1):20. https://doi.org/10.3390/life9010020
Chicago/Turabian StyleMizuuchi, Ryo, and Niles Lehman. 2019. "Limited Sequence Diversity Within a Population Supports Prebiotic RNA Reproduction" Life 9, no. 1: 20. https://doi.org/10.3390/life9010020
APA StyleMizuuchi, R., & Lehman, N. (2019). Limited Sequence Diversity Within a Population Supports Prebiotic RNA Reproduction. Life, 9(1), 20. https://doi.org/10.3390/life9010020