Myalgic Encephalomyelitis (ME) or What? An Operational Definition
Abstract
:1. Introduction
2. Method
3. Results
3.1. Characteristics
3.1.1. An Epidemic and an Endemic Form
3.1.2. An Often Sudden, Sometimes Gradual, Onset
3.1.3. An Acute and a Chronic Phase
3.1.4. Not Just Like Other Post-Viral Fatigue States
3.1.5. A Prolonged Relapsing Course
3.1.6. Diurnal Variability of Symptoms
3.2. Symptoms
3.2.1. Muscle Fatigability/Prolonged Muscle Weakness after Exertion is a Mandatory Feature of ME
3.2.2. Neurological Disturbance Is an Essential Feature of ME
3.2.3. ME Often Coincides with a Number of Symptoms Related to Other Body Systems
3.2.4. ME Is Often Accompanied by Emotional Problems, but Is Not Caused by Those Symptoms
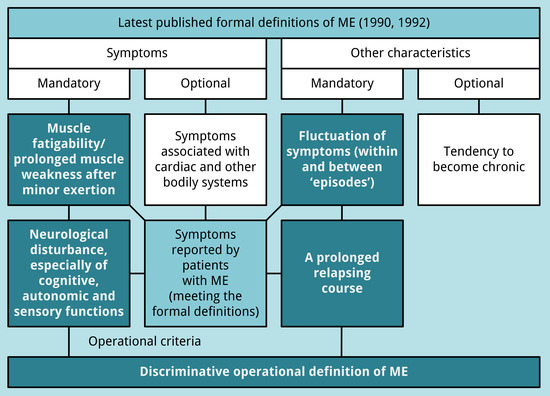
3.3. An Operational Definition of ME
- muscle fatigability/prolonged muscle weakness after trivial exertion;
- neurological disturbance (see Table 3):
- at least one symptom related to cognitive dysfunction or one unclassified neurological symptom;
- at least one symptom related to autonomic dysfunction; and
- at least two symptoms related to sensory dysfunction;
- fluctuation of symptoms (within and between “episodes”); and
- a prolonged relapsing course.
4. Discussion
5. Conclusions
- muscle fatigability/prolonged muscle weakness after trivial exertion;
- neurological disturbance, especially of cognitive, autonomic and sensory functions;
- fluctuation of symptoms (within and between ‘episodes’); and
- a prolonged relapsing course.
Acknowledgments
Conflicts of Interest
References
- Ramsay, A.M. Postviral Fatigue Syndrome—The Saga of Royal Free Disease, 1st ed.; Gower Medical Publishing (for the Myalgic Encephalomyelitis Association): London, UK, 1986. [Google Scholar]
- Dowsett, E.G. Myalgic encephalomyelitis, or what? Lancet 1988, 332, 100–101. [Google Scholar] [CrossRef]
- Dowsett, E.G.; Ramsay, A.M.; McCartney, R.A.; Bell, E.J. Myalgic Encephalomyelitis—A persistent enteroviral infection? Postgrad. Med. J. 1990, 66, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.M.; Dowsett, E.G. Myalgic Encephalomyelitis: Then and now, an epidemiological introduction. In The Clinical and Scientific Basis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Hyde, B.M., Goldstein, J., Levine, P., Eds.; The Nightingale Research Foundation: Ottawa, ON, Canada, 1992; pp. 81–84. [Google Scholar]
- Acheson, E.D. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am. J. Med. 1959, 26, 569–595. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, A.G. Epidemiological Study on an Epidemic, Diagnosed as Poliomyelitis, Occurring among the Personnel of Los Angeles County General Hospital during the Summer of 1934. Available online: https://babel.hathitrust.org/cgi/pt?id=mdp.39015022082260;view=1up;seq=617 (accessed on 3 August 2018).
- Acheson, D.E. A new clinical entity? Lancet 1956, 267, 789–790. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases, Eighth Revision (ICD-8): I (Code 323): 158; WHO: Geneva, Switzerland, 1967. [Google Scholar]
- World Health Organization. International Classification of Diseases, Tenth Revision (ICD-10): G93.3; WHO: Geneva, Switzerland, 1992; Available online: http://apps.who.int/classifications/icd10/browse/2016/en#/G90-G99 (accessed on 7 September 2018).
- Holmes, G.P.; Kaplan, J.E.; Gantz, N.M.; Komaroff, A.L.; Schonberger, L.B.; Straus, S.E.; Jones, J.F.; Dubois, R.E.; Cunningham-Rundles, C.; Pahwa, S.; et al. Chronic fatigue syndrome: A working case definition. Ann. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.; Dobbins, J.G.; Komaroff, A.L. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Hickie, I.; Hadzi-Pavlovic, D.; Wakefield, D.; Parker, G.; Straus, S.E.; Dale, J.; McCluskey, D.; Hinds, G.; Brickman, A.; et al. What is chronic fatigue syndrome? Heterogeneity within an international multicentre study. Aust. N. Z. J. Psychiatry 2001, 35, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Twisk, F.N.M. Myalgic Encephalomyelitis, chronic fatigue syndrome, and Systemic Exertion Intolerance Disease: Three distinct clinical entities. Challenges 2018, 9, 19. [Google Scholar] [CrossRef]
- The Medical Staff of the Royal Free Hospital. An outbreak of Encephalomyelitis in the Royal Free Hospital Group, London, in 1955. Br. Med. J. 1957, 2, 895–904. [Google Scholar] [CrossRef]
- Ramsay, A.M. Encephalomyelitis in north west London; an endemic infection simulating poliomyelitis and hysteria. Lancet 1957, 270, 1196–1200. [Google Scholar] [CrossRef]
- Parish, J.G. Early outbreaks of ‘epidemic neuromyasthenia’. Postgrad. Med. J. 1978, 54, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, B.; Sigurjonsson, J.; Sigurdsson, J.H.; Thorkelsson, J.; Gudmundsson, K.R. A disease epidemic in Iceland simulating poliomyelitis. Am. J. Hyg. 1950, 52, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Damrongvachiraphan, D.; Hunnell, J.; Bartgis, L.; Evans, M.; Brown, A. Myalgic Encephalomyelitis: Case definitions. Auton. Control Physiol. State Funct. 2012, 1, 1–14. [Google Scholar] [CrossRef]
- Jason, L.A.; Brown, A.; Clyne, E.; Bartgis, L.; Evans, M.; Brown, M. Contrasting case definitions for chronic fatigue syndrome, Myalgic Encephalomyelitis/chronic fatigue syndrome and Myalgic Encephalomyelitis. Eval. Health Prof. 2012, 35, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Sunnquist, M.; Jason, L.A.; Nehrke, P.; Goudsmit, E.M. A comparison of case definitions for Myalgic Encephalomyelitis and chronic fatigue syndrome. J. Chronic Dis. Manag. 2017, 2, 1013. [Google Scholar] [PubMed]
- Underhill, R.A. Myalgic encephalomyelitis, chronic fatigue syndrome: An infectious disease. Med. Hypotheses 2015, 85, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, V.; Fang, Y.; Calabrese, L.; Sahgal, V.; Yue, G.H. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin. Neurophysiol. 2004, 115, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, S.M.; MacHale, S.M.; Cavanagh, J.T.; O′Carroll, R.E.; Goodwin, G.M. The difference in patterns of motor and cognitive function in chronic fatigue syndrome and severe depressive illness. Psychol. Med. 2000, 30, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, L.; Wood, L.; Behan, W.M.; Maclaren, W.M. Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur. J. Neurol. 1999, 6, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, K.Y.; White, P.D. Strength and physiological response to exercise in patients with chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 2000, 69, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J. For the 1000 Norms Project Consortium. Normative reference values for strength and flexibility of 1000 children and adults. Neurology 2017, 88, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.K.; Soer, R.; Krijnen, W.P.; Reneman, M.; van der Schans, C.P. Reference values for isometric muscle force among workers for the Netherlands: A comparison of reference values. BMC Sports Sci. Med. Rehabil. 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; de Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International consensus criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.W.M.; Lloyd, A.R. A controversial consensus—Comment on article by Broderick et al. J. Intern. Med. 2012, 271, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, E.G.; Goudsmit, E.; Macintyre, A.; Shepherd, C.B. London criteria for ME. In Report from The National Task Force on Chronic Fatigue Syndrome (CFS), Post Viral Fatigue Syndrome (PVFS), Myalgic Encephalomyelitis (ME); Westcare: Bristol, UK, 1994; pp. 96–98. [Google Scholar]
- Hyde, B. A New and Simple Definition of Myalgic Encephalomyelitis and a New Simple Definition of Chronic Fatigue Syndrome & A Brief History of Myalgic Encephalomyelitis and an Irreverent History of Chronic Fatigue Syndrome. In Proceedings of the Invest in ME Conference 2006, London, UK, 12 May 2006; Available online: http://www.imet.ie/imet_documents/BYRON_HYDE_little_red_book.pdf (accessed on 12 May 2016).

| Definition of ME (1990) |
|---|
| “A syndrome commonly initiated by respiratory and/or gastro-intestinal infection but an insidious or more dramatic onset following neurological, cardiac or endocrine disability occurs. |
The pathognomonic features (of ME) are:
|
| Other characteristics include [..] a prolonged relapsing course and variation in intensity of symptoms within and between episodes, tending to chronicity.” |
| Definition of ME (1992) |
|---|
| “A syndrome initiated by a viral infection commonly described as a respiratory/gastro intestinal illness but a gradual or more dramatic onset following neurological, cardiac or endocrine disability is recognised. The cardinal features, in a patient who has previously been physically and mentally fit, with a good work record are:
|
| Cognitive Dysfunction |
|
| Autonomic Dysfunction |
|
| Sensory Dysfunction |
|
| Motor Dysfunction |
|
| Unclassified Neurological Symptoms |
|
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twisk, F. Myalgic Encephalomyelitis (ME) or What? An Operational Definition. Diagnostics 2018, 8, 64. https://doi.org/10.3390/diagnostics8030064
Twisk F. Myalgic Encephalomyelitis (ME) or What? An Operational Definition. Diagnostics. 2018; 8(3):64. https://doi.org/10.3390/diagnostics8030064
Chicago/Turabian StyleTwisk, Frank. 2018. "Myalgic Encephalomyelitis (ME) or What? An Operational Definition" Diagnostics 8, no. 3: 64. https://doi.org/10.3390/diagnostics8030064
APA StyleTwisk, F. (2018). Myalgic Encephalomyelitis (ME) or What? An Operational Definition. Diagnostics, 8(3), 64. https://doi.org/10.3390/diagnostics8030064