Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review
Abstract
:1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Study Design
2.2. Protocol and Registration
2.3. Eligibility Criteria
2.4. Search and Data Sources
2.5. Data Screening
2.6. Data Charting Process
2.7. Data Items and Extraction
2.8. Appraisal of Individual Sources of Evidence
2.9. Synthesis of Results
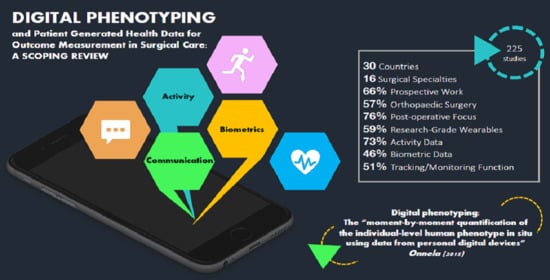
3. Results
3.1. Initial Evaluation and Selection of Studies
3.2. Study Characteristics
3.3. Clinical Characteristics
3.4. Technological/Data Characteristics
3.5. Functional Characteristics
4. Discussion
4.1. Limitations
4.2. Clinical Sphere
Future Scope
4.3. Technological/Data Sphere
Future Scope
4.4. Interpersonal Sphere
Future Scope
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| SECTI.ON | ITEM | PRISMA-ScR CHECKLIST ITEM | REPORTED ON PAGE |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 3 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 4 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 4 |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 4 |
| Search | 8 | Present the full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 4; Appendix B |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 4 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 5 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 5 |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | n/a; Comment on page 5. |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 6 |
| RESULTS | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 6 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 6 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | n/a |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 6,7 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 6,7 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 7–11 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 7 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 11 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 11 |
Appendix B
Appendix C
| Title | Author | Year | Country | Study Design | Surgical Specialty | Pathway Phase | Technology Type | Data Type | Function |
|---|---|---|---|---|---|---|---|---|---|
| Physical activity monitors can be successfully implemented to assess Perioperative activity in urologic surgery | Agarwal, D. K., et al. | 2018 | USA | Feasibility/Validity | Urologic | Pre, Post | CGW | Activity | F |
| Reliability of Physical Activity Measures During Free-Living Activities in People After Total Knee Arthroplasty | Almeida, G. J., et al. | 2016 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | TM, O |
| Responsiveness of Physical Activity Measures Following Exercise Programs after Total Knee Arthroplasty | Almeida, G. J., et al. | 2017 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | O |
| Validity of physical activity measures in individuals after total knee arthroplasty | Almeida, G. J., et al. | 2015 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | F |
| Kinematic and clinical evaluation of shoulder function after primary and revision reverse shoulder prostheses | Alta, T. D., et al. | 2011 | Netherlands | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| The active and passive kinematic difference between primary reverse and total shoulder prostheses | Alta, T. D., et al. | 2014 | Netherlands | Original Prospective | Orthopaedics | Post | RGW | Biometrics | O |
| Long-term clinical evaluation of the automatic stance-phase lock-controlled prosthetic knee joint in young adults with unilateral above-knee Amputation | Andrysek, J., et al. | 2017 | Canada | Original Prospective | Orthopaedics | Post | CGW | Activity, Biometrics | TM |
| Mobile Phone-Connected Wearable Motion Sensors to Assess Postoperative Mobilization | Appelboom, G., et al. | 2015 | USA | Original Prospective | Neurosurgery | Post | CGW | Activity | F |
| Monitoring activity of hip injury patients (MoHIP): a sub-study of the World Hip Trauma Evaluation observational cohort study | Armitage, L. C., et al. | 2020 | UK | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| High Plantar Force Loading After Achilles Tendon Rupture Repair with Early Functional Mobilization | Aufwerber, S., et al. | 2019 | Sweden | Original Prospective | Orthopaedics | Post | CGW | Activity | P |
| Psychological factors are associated with return to Pre-injury levels of sport and physical activity after ACL reconstruction | Baez, S. E., et al. | 2020 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Feasibility of low-cost accelerometers in measuring functional recovery after major oncologic surgery | Barkley, R., et al. | 2019 | USA | Feasibility/Validity | Surgical Oncology | Pre, Post | CGW | Activity | F |
| Assessment of a SP app (Capstesia) for measuring pulse Pressure variation: agreement between two methods: A Cross-sectional study | Barrachina, B., et al. | 2017 | Spain | Feasibility/Validity | General Surgery | Peri | SP | Biometrics | F |
| Physical Activity, Quality of Life and Body Image of Candidates to Bariatric Surgery | Barreto, B. L. M., et al. | 2018 | Brazil | Original Prospective | Bariatric | Post | CGW | Activity | O |
| Cementless THA for the treatment of osteonecrosis at 10-year follow-up: have we improved compared to cemented THA? | Bedard, N. A., et al. | 2013 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Functional outcome analysis of operatively treated malleolar fractures | Belcher, G. L., et al. | 1997 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Changes in prospectively collected longitudinal patient-generated health data are associated with short-term patient-reported outcomes after total joint arthroplasty: a pilot study | Bendich, I., et al. | 2019 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | RP |
| Activity levels and polyethylene wear of patients 10 years Post hip replacement | Bennett, D., et al. | 2008 | UK | Original Cross-sectional | Orthopaedics | Post | CGW | Activity | RP |
| Geriatric rehabilitation after hip fracture. Role of body-fixed sensor measurements of physical activity | Benzinger, P., et al. | 2014 | Germany | Original Cross-sectional | Orthopaedics | Post | CGW | Activity | F |
| Postoperative quality-of-life assessment in patients with spine metastases treated with long-segment pedicle-screw fixation | Bernard, F., et al. | 2017 | France | Original Retrospective | Neurosurgery | Post | CGW | Activity | TM |
| What are the functional outcomes of endoprosthestic reconstructions after tumor resection? | Bernthal, N. M., et al. | 2015 | USA | Original Prospective | Surgical Oncology | Post | RGW | Activity, Biometrics | P |
| Pervasive wearable device for free tissue transfer monitoring based on advanced data analysis: clinical study report | Berthelot, M., et al. | 2019 | UK | Original Prospective | Breast | Peri | RGW | Biometrics | F |
| Machine Learning Algorithms Can Use Wearable Sensor Data to Accurately Predict Six-Week Patient-Reported Outcome Scores Following Joint Replacement in a Prospective Trial | Bini, S. A., et al. | 2019 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity, Biometrics | P |
| Monitoring of Postoperative Bone Healing Using Smart Trauma-Fixation Device with Integrated Self-Powered Piezo-Floating-Gate Sensors | Borchani, W., et al. | 2015 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Cross-sectional assessment of daily physical activity in chronic obstructive Pulmonary disease lung transplant patients | Bossenbroek, L., et al. | 2009 | Netherlands | Original Cross-sectional | Transplant | Post | CGW | Activity, Biometrics | TM |
| Changes in physical activity and health-related quality of life during the first year after total knee arthroplasty | Brandes, M., et al. | 2011 | Germany | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | TM |
| Quantity versus quality of gait and quality of life in patients with osteoarthritis | Brandes, M., et al. | 2008 | Germany | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | F |
| Impact of a tailored activity counselling intervention during inpatient rehabilitation after knee and hip arthroplasty—an explorative RCT | Brandes, M., et al. | 2018 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity | O |
| Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: a clinical validation study | Breteler, M. J.M. M., et al. | 2018 | Netherlands | Original Prospective | General Surgery | Post | RGW | Activity, Biometrics | F |
| Are current wireless monitoring systems capable of detecting adverse events in high-risk surgical patients? A descriptive study | Breteler, M. J. M., et al. | 2020 | Netherlands | Original Cross-sectional | General Surgery | Post | RGW | Biometrics | F |
| Vital Signs Monitoring with Wearable Sensors in High-risk Surgical Patients: A Clinical Validation Study | Breteler, M. J. M., et al. | 2020 | Netherlands | Original Cross-sectional | General Surgery | Post | RGW | Biometrics | TM |
| Novel positioning sensor with real-time feedback for improved Postoperative positioning: pilot study in control subjects | Brodie, F. L., et al. | 2017 | USA | Original Prospective | Ophthalmology | Peri | CGW | Biometrics | F |
| Validity and reliability of measurements obtained with an “activity monitor” in people with and without a transtibial Amputation | Bussmann, H. B., et al. | 1998 | Netherlands | Feasibility/Validity | Orthopaedics | Post | RGW | Activity, Biometrics | F |
| Validity of the prosthetic activity monitor to assess the duration and spatio-temporal characteristics of prosthetic walking | Bussmann, J. B., et al. | 2004 | Netherlands | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Ambulatory accelerometry to quantify motor behaviour in patients after failed back surgery: a validation study | Bussmann, J. B., et al. | 1998 | Netherlands | Feasibility/Validity | Neurosurgery | Post | RGW | Activity, Biometrics | F |
| Inertial Sensor-Based Gait and Attractor Analysis as Clinical Measurement Tool: Functionality and Sensitivity in Healthy Subjects and Patients with Symptomatic Lumbar Spinal Stenosis | Byrnes, S. K., et al. | 2018 | Switzerland | Feasibility/Validity | Neurosurgery | Post | RGW | Biometrics | O |
| Cardiac Surgery Rehabilitation System (CSRS) for a Personalized Support to Patients | Caggianese, G., et al. | 2017 | Italy | Original Prospective | Cardiothoracic | Post | CGW | Activity | TM |
| Clinical evaluation of a mobile sensor-based gait analysis method for outcome measurement after knee arthroplasty | Calliess, T., et al. | 2014 | Germany | Feasibility/Validity | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | F |
| Higher pyruvate levels after Achilles tendon rupture surgery could be used as a prognostic biomarker of an improved patient outcome | Capone, G., et al. | 2020 | Sweden | Original Prospective | Orthopaedics | Post | CGW | Activity | P |
| Wearable Technology-A Pilot Study to Define “Normal” Postoperative Recovery Trajectories | Carmichael, H., et al. | 2019 | USA | Original Prospective | General Surgery | Pre, Post | CGW | Activity | TM |
| Patterns of physical activity and sedentary behavior after Bariatric: an observational study | Chapman, N., et al. | 2014 | Australia | Original Prospective | Bariatric | Post | RGW | Activity, Biometrics | O |
| Data Collection and Analysis Using Wearable Sensors for Monitoring Knee Range of Motion after Total Knee Arthroplasty | Chiang, C. Y., et al. | 2017 | Taiwan | Original Prospective | Orthopaedics | Post | RGW | Activity | F |
| Feasibility and Preliminary Outcomes of a Physical Therapist-Administered Physical Activity Intervention After Total Knee Replacement | Christiansen, M. B., et al. | 2019 | USA | Feasibility/Validity | Orthopaedics | Post | CGW | Activity | F |
| An Assessment of Physical Activity Data Collected via a Smartphone App and a Smart Band in Breast Cancer Survivors: Observational Study | Chung, I. Y., et al. | 2019 | South korea | Original Prospective | Surgical Oncology | Post | SP, CGW | Activity, Biometrics | F |
| Inertial sensor-based measures of gait symmetry and repeatability in people with unilateral lower limb Amputation | Clemens, S., et al. | 2020 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Use of a wrist-mounted device for continuous outpatient physiologic monitoring after transsphenoidal surgery: a pilot study | Cole, T. S., et al. | 2019 | USA | Original Prospective | Oromaxillofacial | Post | CGW | Activity, Biometrics | F |
| Understanding the Capacity for Exercise in Post-Bariatric Patients | Coleman, K. J., et al. | 2017 | USA | Original Prospective | Bariatric | Post | CGW | Activity | F, TM |
| A multicomponent intervention to decrease sedentary time during hospitalization: a quasi-exPerimental pilot study | Conijn, D., et al. | 2020 | Netherlands | Original Prospective | Vascular, Transplantation | Post | CGW | Activity | F |
| Digital Phenotyping in Patients with Spine Disease: A Novel Approach to Quantifying Mobility and Quality of Life | Cote, D. J., et al. | 2019 | USA | Original Prospective | Neurosurgery | Post | SP | Activity, Communication | TM |
| Late effects of a brief psychological intervention in patients with intermittent claudication in a randomized clinical trial | Cunningham, M. A., et al. | 2013 | Australia | Original Prospective | Vascular | Post | Unknown | Activity | P |
| Daily Physical Activity in Total Hip Arthroplasty Patients Undergoing Different Surgical Approaches A Cohort Study | Engdal, M., et al. | 2017 | Norway | Original Prospective | Orthopaedics | Post | RGW | Activity, Biometrics | TM |
| Validation of the Fitbit Flex in an Acute Post-Cardiac Surgery Patient Population | Daligadu, J., et al. | 2018 | Canada | Feasibility/Validity | Cardiothoracic | Post | CGW | Activity | F |
| Association of Wearable Activity Monitors with Assessment of Daily Ambulation and Length of Stay Among Patients Undergoing Major Surgery | Daskivich, T. J., et al. | 2019 | USA | Original Prospective | Cardiothoracic, General Surgery, Bariatric | Post | CGW | Activity | F |
| Are patients with knee osteoarthritis and patients with knee joint replacement as physically active as healthy persons? | Daugaard, R., et al. | 2018 | Denmark | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Physical Activity Levels During Acute Inpatient Admission After Hip Fracture are Very Low | Davenport, S. J., et al. | 2014 | Australia | Original Cross-sectional | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | TM |
| Feasibility of real-time location systems in monitoring recovery after major abdominal surgery | Dorrell, R. D., et al. | 2017 | USA | Original Prospective | General Surgery | Post | RGW | Activity | TM |
| Continuous Versus Intermittent Vital Signs Monitoring Using a Wearable, Wireless Patch in Patients Admitted to Surgical Wards: Pilot Cluster Randomized Controlled Trial | Downey, C., et al. | 2018 | UK | Feasibility/Validity | General Surgery | Post | RGW | Biometrics | F |
| Distribution of arm velocity and frequency of arm usage during daily activity: objective outcome evaluation after shoulder surgery | Duc, C., et al. | 2013 | Switzerland | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | TM |
| Objective evaluation of cervical spine mobility after surgery during free-living activity | Duc, C., et al. | 2013 | Belgium | Feasibility/Validity | Neurosurgery | Post | RGW | Biometrics | TM |
| Ambulation monitoring of transtibial Amputation subjects with patient activity monitor versus pedometer | Dudek, N. L., et al. | 2008 | Canada | Feasibility/Validity | Orthopaedics | Post | CGW | Activity | F |
| Evaluating patients’ walking capacity during hospitalization for lung cancer resection | Esteban, P. A., et al. | 2017 | Spain | Original Cross-sectional | Cardiothoracic | Post | CGW | Activity | TM |
| Activity and socket wear in the Charnley low-friction arthroplasty | Feller, J. A., et al. | 1994 | Australia | Original Retrospective | Orthopaedics | Post | Unknown | Activity | TM |
| Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? | Ferriolli, E., et al. | 2012 | UK | Original Cross-sectional | General Surgery | Post | RGW | Activity | F |
| A feasibility study of an unsupervised, Pre-operative exercise program for adults with lung cancer | Finley, D. J., et al. | 2020 | USA | Feasibility/Validity | Cardiothoracic | Pre | CGW | Activity, Biometrics | F |
| Differences in Preferred walking speeds in a gait laboratory compared with the real world after total hip replacement | Foucher, K. C., et al. | 2010 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Pilot study of methods to document quantity and variation of independent patient exercise and activity after total knee arthroplasty | Franklin, P. D., et al. | 2006 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | F, TM |
| Improvements in Objectively Measured Activity Behaviors Do Not Correlate with Improvements in Patient-Reported Outcome Measures Following Total Knee Arthroplasty | Frimpong, E., et al. | 2020 | South Africa | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | P |
| Prospective study of physical activity and quality of life in Japanese women undergoing total hip arthroplasty | Fujita, K., et al. | 2013 | Japan | Original Cross-sectional | Orthopaedics | Pre, Post | RGW | Activity | TM |
| Effects of cycle ergometer use in early mobilization following cardiac surgery: a randomized controlled trial | Gama Lordello, G. G., et al. | 2020 | Brazil | Original Prospective | Cardiothoracic | Post | CGW | Activity | P |
| Enhancing patient mobility following cesarean-delivery—the efficacy of an improved Postpartum protocol assessed with pedometers | Ganer Herman, H., et al. | 2020 | Israel | Original Prospective | Obstetrics/Gynecology | Post | CGW | Activity | P |
| Assessment and Post-Intervention recovery following surgery for Lumbar Disc Herniation based on objective gait metrics from wearable devices using the Gait Posture index: GPi™ | Ghent, F., et al. | 2020 | Australia | Feasibility/Validity | Neurosurgery | Pre, Post | CGW | Activity, Biometrics | TM |
| Physical activity patterns of patients immediately after lumbar surgery | Gilmore, S. J., et al. | 2019 | Australia | Original Prospective | Neurosurgery | Post | RGW | Activity | P |
| Assessing the utility of an IoS application in the Perioperative care of spine surgery patients: the NeuroPath Pilot study | Glauser, G., et al. | 2019 | USA | Feasibility/Validity | Neurosurgery | Pre, Post | SP | Activity, Communication | F |
| A Step in the Right Direction: Body Location Determines Activity Tracking Device Accuracy in Total Knee and Hip Arthroplasty Patients | Goel, R., et al. | 2020 | USA | Original Prospective | Orthopaedics | Post | CGW | Biometrics | TM |
| Comparative study of the activity of total hip arthroplasty patients and normal subjects | Goldsmith, A. A., et al. | 2001 | UK | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| CAPACITY: A physical activity self-management program for patients undergoing surgery for lung cancer, a phase I feasibility study | Granger, C. L., et al. | 2018 | Australia | Original Prospective | Cardiothoracic | Pre, Post | CGW | Activity | F |
| Accelerometery as a measure of modifiable physical activity in high-risk elderly Preoperative patients: a prospective observational pilot study | Grimes, L., et al. | 2019 | UK | Original Prospective | General Surgery | Pre | CGW | Activity | F, TM |
| Does the Femoral Head Size in Hip Arthroplasty Influence Lower Body Movements during Squats, Gait and Stair Walking? A Clinical Pilot Study Based on Wearable Motion Sensors | Grip, H., et al. | 2019 | Sweden | Feasibility/Validity | Orthopaedics | Post | RGW | Activity, Biometrics | F |
| Assessment of objective ambulation in lower extremity sarcoma patients with a continuous activity monitor: rationale and validation | Gundle, K. R., et al. | 2014 | USA | Feasibility/Validity | Surgical Oncology | Post | RGW | Activity | F |
| Remote Gait Analysis Using Wearable Sensors Detects Asymmetric Gait Patterns in Patients Recovering from ACL Reconstruction | Gurchiek, R. D., et al. | 2019 | USA | Original Cross-sectional | Orthopaedics | Post | RGW | Biometrics | F |
| Open-Source Remote Gait Analysis: A Post-Surgery Patient Monitoring Application | Gurchiek, R. D., et al. | 2019 | USA | Feasibility/Validity | Orthopaedics | Post | SP, RGW | Activity, Biometrics | F |
| Physical performance and self-report outcomes associated with use of passive, adaptive, and active prosthetic knees in persons with unilateral, transfemoral Amputation: Randomized crossover trial | Hafner, B. J. and R. L. Askew | 2015 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Using MEMS-based inertial sensor with ankle foot orthosis for telerehabilitation and its clinical evaluation in brain injuries and total knee replacement patients | Han, S. L., et al. | 2016 | Taiwan | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | F |
| Do activity levels increase after total hip and knee arthroplasty? | Harding, P., et al. | 2014 | Australia | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | TM |
| Knee arthroplasty: a cross-sectional study assessing energy expenditure and activity | Hayes, D. A., et al. | 2011 | Australia | Original Cross-sectional | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | P |
| Wearable Technology in the Perioperative Period: Predicting Risk of Postoperative Complications in Patients Undergoing Elective Colorectal | Hedrick, T. L., et al. | 2020 | USA | Original Prospective | Colorectal | Pre, Post | CGW | Activity | RP |
| Detecting Postural transitions: a robust wavelet-based approach | Hemmati, S. and E. Wade | 2016 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | F |
| Low validity of the Sensewear Pro3 activity monitor compared to indirect calorimetry during simulated free living in patients with osteoarthritis of the hip | Hermann, A., et al. (2014). | 2014 | Denmark | Original Cross-sectional | Orthopaedics | Pre, Post | RGW | Biometrics | F |
| Clinical outcome and physical activity measured with StepWatch 3 (TM) Activity Monitor after minimally invasive total hip arthroplasty | Holl, S., et al. | 2018 | Germany | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | TM |
| Interaction between physical activity and continuous-flow left ventricular assist device function in outpatients | Hu, S.X., et al. | 2013 | Australia | Original Prospective | Cardiothoracic | Post | RGW | Activity, Biometrics | TM, P |
| 2009 Marshall Urist Young Investigator Award: how often do patients with high-flex total knee arthroplasty use high flexion? | Huddleston, J. I., et al. | 2009 | USA | Original Cross-sectional | Orthopaedics | Post | RGW | Activity | TM, P |
| Tri-axial accelerometer analysis techniques for evaluating functional use of the extremities | Hurd, W.J., et al. | 2013 | USA | Original Prospective | Orthopaedics | Pre | RGW | Activity | F |
| Patient-Reported and Objectively Measured Function Before and After Reverse Shoulder Arthroplasty | Hurd, W.J., et al. | 2018 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | F, TM |
| A Smart Assistance Solution for Remotely Monitoring the Orthopaedic Rehabilitation Process Using Wearable Technology: re.flex System | Ianculescu, M., et al. | 2019 | Romania | Feasibility/Validity | Orthopaedics | Post | CGW | Activity | F |
| Physical activity patterns and function 3 months after arthroscopic partial meniscectomy | Ilich, S.S., et al. | 2012 | Australia | Original Prospective | Neurosurgery | Post | RGW | Activity | TM |
| Objective evaluation of Postoperative changes in real-life activity levels in the Postoperative course of lumbar spinal surgery using wearable trackers | Inoue, M., et al. | 2020 | Japan | Original Prospective | Neurosurgery | Post | RGW | Activity | TM |
| HipGuard: A Wearable Measurement System for Patients Recovering from a Hip Operation | Iso-Ketola, P., et al. | 2008 | Finland | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Upright Time and Sit-To-Stand Transition Progression After Total Hip Arthroplasty: An Inhospital Longitudinal Study | Jeldi, A. J., et al. | 2016 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Metal ion concentrations after metal-on-metal hip arthroplasty are not correlated with habitual physical activity levels | Jelsma, J., et al. | 2019 | Netherlands | Original Prospective | Orthopaedics | Post | RGW | Activity | P |
| Association of Daily Step Count with the Prolonged Air Leak in Thoracic Surgery Patients | Kavurmaci, Ö., et al. | 2020 | Turkey | Original Cross-sectional | Cardiothoracic | Post | Unknown | Activity | P |
| The Usefulness of a Wearable Device in Daily Physical Activity Monitoring for the Hospitalized Patients Undergoing Lumbar Surgery | Kim, D. H., et al. | 2019 | South Korea | Original Prospective | Neurosurgery | Post | CGW | Activity, Biometrics | TM, P |
| Associations between physical activity and mental health among Bariatric surgical candidates | King, W. C., et al. | 2013 | USA | Original Cross-sectional | Bariatric | Pre | RGW | Activity | TM, RP, O |
| Seasonal Variation in Physical Activity among Preoperative Patients with Lung Cancer Determined Using a Wearable Device | Kong, S., et al. | 2020 | South Korea | Original Cross-sectional | Cardiothoracic | Pre | CGW | Activity | TM |
| Gamified 3D Orthopaedic Rehabilitation using Low Cost and Portable Inertial Sensors | Kontadakis, G., et al. | 2017 | Greece | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | F |
| Relationship Between Physical Activity and Clinical Outcomes After ACL Reconstruction | Kuenze, C., et al. | 2019 | USA | Original Cross-sectional | Orthopaedics | Post | RGW | Activity | F, TM |
| Gait Pattern Recognition Using a Smartwatch Assisting Postoperative Physiotherapy | Kyritsis, A. I., et al. | 2019 | Switzerland | Original Prospective | Orthopaedics | Post | CGW | Biometrics | F |
| Gait Recognition with Smart Devices Assisting Postoperative Rehabilitation in a Clinical Setting | Kyritsis, A. I., et al. | 2018 | Switzerland | Feasibility/Validity | Orthopaedics | Post | CGW | Biometrics | F |
| Recovery of mobility after knee arthroplasty—Expected rates and influencing factors | Lamb, S. E. and H. Frost | 2003 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Physical activity is unrelated to cognitive performance in Pre-Bariatric patients | Langenberg, S., et al. | 2015 | Germany | Original Prospective | Bariatric | Pre | RGW | Activity, Biometrics | RP, P, O |
| Physical activity in daily life 1 year after lung transplantation | Langer, D., et al. | 2009 | Belgium | Original Prospective | Transplant | Post | RGW | Activity, Biometrics | TM |
| Predicting physical activity recovery after hip and knee arthroplasty? A longitudinal cohort study | Lebleu, J., et al. | 2019 | Belgium | Original Cross-sectional | Orthopaedics | Post | CGW | Activity | TM, P |
| iHandU: Towards the Validation of a Wrist Rigidity Estimation for Intraoperative DBS Electrode Position Optimization | Lopes, E. M., et al. | 2019 | Portugal | Feasibility/Validity | Neurosurgery | Peri | RGW | Biometrics | F |
| Adherence to a pedometer-based physical activity intervention following kidney transplant and impact on metabolic parameters | Lorenz, E. C., et al. | 2015 | USA | Original Prospective | Transplant | Post | CGW | Activity | F, TM, P |
| Financial Incentives and Health Coaching to Improve Physical Activity Following Total Knee Replacement: A Randomized Controlled Trial | Losina, E., et al. | 2018 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | F, TM, P |
| Fitbit step counts during inpatient recovery from cancer surgery as a Predictor of readmission | Low, C. A., et al. | 2017 | USA | Original Prospective | Surgical Oncology | Post | CGW | Activity | TM, RP |
| Is Activity Tracker-Measured Ambulation an Accurate and Reliable Determinant of Postoperative Quality of Recovery? A Prospective Cohort Validation Study | Massouh, F., et al. | 2019 | Canada | Original Prospective | Obstetrics/Gynecology | Post | CGW | Activity | F |
| Relationship between body mass index and activity in hip or knee arthroplasty patients | McClung, C. D., et al. | 2000 | USA | Original Cross-sectional | Orthopaedics | Post | Unknown | Activity | TM, RP |
| Patient-Generated Actigraphy Data as a Novel Outcomes Instrument in Carpal Tunnel Syndrome | McMahon, H. A., et al. | 2020 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity, Biometrics | F |
| Use of the pedometer in the evaluation of the effects of rehabilitation treatment on deambulatory autonomy in patients with lower limb arthroplasty during hospital rehabilitation: long-term Postoperative outcomes | Melchiorri, G., et al. | 2020 | Italy | Original Cross-sectional | Orthopaedics | Post | CGW | Activity | F, TM |
| Physical Function and Pre-Amputation Characteristics Explain Daily Step Count after Dysvascular Amputation | Miller, M. J., et al. | 2019 | USA | Original Cross-sectional | Vascular | Post | RGW | Activity | F, TM, RP |
| Evaluation of respiratory status and mandibular movement after total temporomandibular joint replacement in patients with rheumatoid arthritis | Mishima, K., et al. | 2003 | Japan | Original Prospective | Oromaxillofacial | Pre, Post | RGW | Biometrics | F, TM |
| Real-Time Monitoring of Bone Fracture Recovery by Using Aware, Sensing, Smart, and Active Orthopedic Devices | Mišić, D., et al. | 2018 | Serbia | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi) | Mobbs, R. J., et al. | 2019 | Australia | Feasibility/Validity | Neurosurgery | Post | CGW | Activity, Biometrics | F, RP, P |
| Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study | Mobbs, R. J., et al. | 2016 | Australia | Original Prospective | Neurosurgery | Pre, Post | CGW | Activity, Biometrics | F, TM, RP |
| Outcome of the modified Lapidus procedure for hallux valgus deformity during the first year following surgery: A prospective clinical and gait analysis study | Moerenhout, K., et al. | 2019 | Switzerland | Original Prospective | Orthopaedics | Post | RGW | Biometrics | F, TM |
| Physical Function, Quality of Life, and Energy Expenditure During Activities of Daily Living in Obese, Post-Bariatric, and Healthy Subjects | Monteiro, F., et al. | 2017 | Brazil | Original Prospective | Bariatric | Post | RGW | Activity | F, TM, P |
| Towards a new Concept to the Neurological Recovery for Knee Stabilization after Anterior Cruciate Ligament Reconstruction Based on Surface Electrical Stimulation | Moreno, J. C., et al. | 2008 | Spain | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Duration and frequency of every day activities in total hip patients | Morlock, M., et al. | 2001 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity | F, TM |
| Physical performance in kidney transplanted patients: a study on desert trekking | Mosconi, G., et al. | 2011 | Italy | Original Prospective | Transplant | Post | RGW | Activity, Biometrics | TM |
| Identifying subgroups of community-dwelling older adults and their prospective associations with long-term knee osteoarthritis outcomes | Munugoda, I. P., et al. | 2020 | Australia | Original Prospective | Orthopaedics | Pre | CGW | Activity | TM, RP, P |
| High-grade rotatory knee laxity may be Predictable in ACL injuries | Musahl, V., et al. | 2018 | USA | Original Prospective | Orthopaedics | Pre | RGW | Biometrics | RP, P |
| The effect of patella resurfacing in total knee arthroplasty on functional range of movement measured by flexible electrogoniometry | Myles, C. M., et al. | 2006 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM, P |
| Knee joint functional range of movement prior to and following total knee arthroplasty measured using flexible electrogoniometry | Myles, C. M., et al. | 2002 | UK | Original Prospective | Orthopaedics | Pre, Post | RGW | Biometrics | TM |
| How Many Steps Per Day are Necessary to Prevent Postoperative Complications Following Hepato-Pancreato-Biliary Surgeries for Malignancy? | Nakajima, H., et al. | 2020 | Japan | Original Prospective | Surgical Oncology, General Surgery | Pre | RGW | Activity | RP |
| Assessment of Early Gait Recovery After Anterior Approach Compared to Posterior Approach Total Hip Arthroplasty: A Smartphone Accelerometer-Based Study | Nelms, N. J., et al. | 2019 | USA | Original Prospective | Orthopaedics | Pre, Post | SP | Activity, Biometrics | RP |
| Value of the average basal daily walked distance measured using a pedometer to Predict maximum oxygen consumption per minute in patients undergoing lung resection | Novoa, N. M., et al. | 2011 | Spain | Original Prospective | Cardiothoracic | Pre, Post | CGW | Activity | F, P |
| Influence of major Pulmonary resection on Postoperative daily ambulatory activity of the patients | Novoa, N., et al. | 2009 | Spain | Original Prospective | Cardiothoracic | Pre, Post | CGW | Activity, Biometrics | P |
| A prospective randomised double-blind study of functional outcome and range of flexion following total knee replacement with the NexGen standard and high flexion components | Nutton, R. W., et al. | 2008 | UK | Original Prospective | Orthopaedics | Post | RGW | Activity, Biometrics | TM |
| Does a mobile-bearing, high-flexion design increase knee flexion after total knee replacement? | Nutton, R. W., et al. | 2012 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial | Oosting, E., et al. | 2012 | Netherlands | Feasibility/Validity | Orthopaedics | Pre, Post | CGW | Activity | F |
| User Friendliness of a Wearable Visual Behavior Monitor for Cataract and Refractive Surgery | Pajic, B., et al. | 2020 | Switzerland | Original Prospective | Ophthalmology | Pre | CGW | Biometrics | F |
| Mandibular motion after closed and open treatment of unilateral mandibular condylar process fractures | Palmieri, C., et al. | 1999 | USA | Original Prospective | Oromaxillofacial | Post | RGW | Biometrics | TM |
| Using Smartphones to Capture Novel Recovery Metrics After Cancer Surgery | Panda, N., et al. | 2020 | USA | Original Prospective | Surgical Oncology | Post | SP | Activity | F |
| Wearable activity sensors and early pain after total joint arthroplasty | Patterson, J. T., et al. | 2020 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity, Biometrics | TM, RP |
| Armband activity monitor data do not correlate with reported pain scores in patients receiving vertebroplasty | Peacock, J. G., et al. | 2016 | USA | Original Prospective | Neurosurgery | Post | RGW | Activity, Biometrics | TM, RP |
| Alteration and recovery of arm usage in daily activities after rotator cuff surgery | Pichonnaz, C., et al. | 2015 | Switzerland | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Objectively measured mobilisation is enhanced by a new behaviour support tool in patients undergoing abdominal cancer surgery | Porserud, A., et al. | 2019 | Sweden | Original Prospective | Surgical Oncology | Pre, Post | RGW | Activity | TM |
| Activity and affect: repeated within-participant assessment in people after joint replacement surgery | Powell, R., et al. | 2009 | UK | Original Prospective | Orthopaedics | Post | RGW | Activity | P |
| Continuous Digital Assessment for Weight Loss Surgery Patients | Ramirez, E., et al. | 2020 | USA | Original Prospective | Bariatric | Post | CGW | Biometrics | TM |
| Remote Patient Monitoring Using Mobile Health for Total Knee Arthroplasty: Validation of a Wearable and Machine Learning-Based Surveillance Platform | Ramkumar, P. N., et al. | 2019 | USA | Feasibility/Validity | Orthopaedics | Post | SP, RGW | Activity, Biometrics | TM |
| Walking, Sedentary Time and Health-Related Quality Life Among Kidney Transplant Recipients: An Exploratory Study | Raymond, J., et al. | 2015 | Canada | Original Cross-sectional | Transplant | Post | RGW | Activity, Biometrics | O |
| Dual Mode Gait Sonification for Rehabilitation After Unilateral Hip Arthroplasty | Reh, J., et al. | 2019 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity, Biometrics | TM |
| A prospective randomized comparison of the minimally invasive direct anterior and the transgluteal approach for primary total hip arthroplasty | Reichert, J. C., et al. | 2018 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity | O |
| Physical Activity and Sedentary Behavior in Bariatric Patients Long-Term Post-Surgery | Reid, R. E. R., et al. | 2015 | Canada | Original Prospective | Bariatric | Post | RGW | Activity, Biometrics | TM |
| Physical activity levels after limb salvage surgery are not related to clinical scores-objective activity assessment in 22 patients after malignant bone tumor treatment with modular prostheses | Rosenbaum, D., et al. | 2008 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Multi-segment foot kinematics after total ankle replacement and ankle arthrodesis during relatively long-distance gait | Rouhani, H., et al. | 2012 | Switzerland | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM, O |
| The effect of total knee arthroplasty on joint movement during functional activities and joint range of motion with particular regard to higher flexion users | Rowe, P. J., et al. | 2005 | UK | Original Prospective | Orthopaedics | Pre, Post | RGW | Biometrics | RP |
| Energy Harvesting and Sensing with Embedded Piezoelectric Ceramics in Knee Implants | Safaei, M., et al. | 2018 | USA | Feasibility/Validity | Orthopaedics | Post | RGW | Activity, Biometrics | F |
| Development and validation of a lower-extremity activity scale. Use for patients treated with revision total knee arthroplasty | Saleh, K. J., et al. | 2005 | USA | Feasibility/Validity | Orthopaedics | Post | Unknown | Activity | P |
| Initial ExPerience with Real-Time Continuous Physical Activity Monitoring in Patients Undergoing Spine Surgery | Scheer, J. K., et al. | 2017 | USA | Original Prospective | Neurosurgery | Post | CGW | Activity | TM |
| Validation of Activity Tracking Procedures in Elderly Patients after Operative Treatment of Proximal Femur Fractures | Schmal, H., et al. | 2018 | Denmark | Original Prospective | Orthopaedics | Post | CGW | Activity | O |
| Quantitative assessment of walking activity after total hip or knee replacement | Schmalzried, T. P., et al. | 1998 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Physical activity after outpatient surgery and enhanced recovery for total knee arthroplasty | Schotanus, M. G. M., et al. | 2017 | Netherlands | Original Prospective | Orthopaedics | Post | CGW | Activity | TM, O |
| Step activity monitoring in lumbar stenosis patients undergoing decompressive surgery | Schulte, T. L., et al. | 2010 | Germany | Original Prospective | Neurosurgery | Pre, Post | RGW | Activity | TM |
| Horizontal jumping biomechanics among elite male handball players with and without anterior cruciate ligament reconstruction. An inertial sensor unit-based study | Setuain, I., et al. | 2019 | Spain | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Acceleration and Orientation Jumping Performance Differences Among Elite Professional Male Handball Players with or Without Previous ACL Reconstruction: An Inertial Sensor Unit-Based Study | Setuain, I., et al. | 2015 | Spain | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Optimal Sampling Frequency for Wearable Sensor Data in Arthroplasty Outcomes RGW. A Prospective Observational Cohort Trial | Shah, R. F., et al. | 2019 | USA | Original Prospective | Orthopaedics | Post | CGW | Biometrics | P |
| Step Activity After Surgical Treatment of Ankle Arthritis | Shofer, J. B., et al. | 2019 | USA | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | TM |
| Activity sampling in the assessment of patients with total joint arthroplasty | Silva, M., et al. | 2005 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Dynamic assessment of the wrist after total wrist arthroplasty | Singh, H. P., et al. | 2017 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Dynamic assessment of wrist after proximal row carpectomy and 4-corner fusion | Singh, H. P., et al. | 2014 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Comparison of the clinical and functional outcomes following 3- and 4-corner fusions | Singh, H. P., et al. | 2015 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Quantifying Real-World Upper-Limb Activity Via Patient-Initiated Movement After Nerve Reconstruction for Upper Brachial Plexus Injury | Smith, B. W., et al. | 2019 | USA | Original Prospective | Neurosurgery | Post | RGW | Activity | F |
| The effect of electromagnetic navigation in total knee arthroplasty on knee kinematics during functional activities using flexible electrogoniometry | Smith, J. R., et al. | 2013 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | O |
| A Randomized Study of Exercise and Fitness Trackers in Obese Patients After Total Knee Arthroplasty | Smith, W. A., et al. | 2019 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | O |
| Objective measurement of function following lumbar spinal stenosis decomPression reveals improved functional capacity with stagnant real-life physical activity | Smuck, M., et al. | 2018 | USA | Original Prospective | Neurosurgery | Pre, Post | RGW | Activity, Biometrics | TM |
| Preliminary evidence for physical activity following pelvic exenteration: a pilot longitudinal cohort study | Steffens, D., et al. | 2019 | Australia | Original Prospective | Surgical Oncology | Post | RGW | Activity | TM |
| A Cyber-Physical System for Near Real-Time Monitoring of At-Home Orthopedic Rehabilitation and Mobile-Based Provider-Patient Communications to Improve Adherence: Development and Formative Evaluation | Stevens, T., et al. | 2020 | USA | Feasibility/Validity | Orthopaedics | Post | SP | Activity | F |
| Reliability of the 6-min walking test Smartphone application | Stienen, M. N., et al. | 2019 | Switzerland | Feasibility/Validity | Neurosurgery | Post | SP | Activity | F |
| Wireless Monitoring Program of Patient-Centered Outcomes and Recovery Before and After Major Abdominal Cancer Surgery | Sun, V., et al. | 2017 | USA | Original Prospective | General Surgery | Pre, Post | CGW | Activity | TM |
| Clinical Evaluation of Implant-Supported Removable Partial Dentures with a Stress-Breaking Attachment | Suzuki, Y., et al. | 2017 | Japan | Original Prospective | Oromaxillofacial | Post | CGW | Biometrics | TM, O |
| A Mobile Health Application to Track Patients After Gastrointestinal Surgery: Results from a Pilot Study | Symer, M. M., et al. | 2017 | USA | Feasibility/Validity | Colorectal | Post | CGW | Biometrics | TM |
| Which functional assessments Predict long-term wear after total hip arthroplasty? | Takenaga, R. K., et al. | 2013 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | P |
| Physical Behavior and Function Early After Hip Fracture Surgery in Patients Receiving Comprehensive Geriatric Care or Orthopedic Care-A Randomized Controlled Trial | Taraldsen, K., et al. | 2014 | Norway | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM, P |
| Multiple days of monitoring are needed to obtain a reliable estimate of physical activity in hip-fracture patients | Taraldsen, K., et al. | 2014 | Norway | Original Prospective | Orthopaedics | Post | RGW | Activity, Biometrics | TM, O |
| The long-term effect of being treated in a geriatric ward compared to an orthopaedic ward on six measures of free-living physical behavior 4 and 12 months after a hip fracture—a randomised controlled trial | Taraldsen, K., et al. | 2014 | Norway | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| John Charnley Award: Randomized Clinical Trial of Direct Anterior and MiniPosterior Approach THA: Which Provides Better Functional Recovery? | Taunton, M. J., et al. | 2018 | USA | Original Prospective | Orthopaedics | Post | RGW | Activity | O |
| Quantified-Self for Obesity: Physical Activity Behaviour Sensing to Improve Health Outcomes | Taylor, D., et al. | 2016 | UK | Original Prospective | Bariatric | Pre, Post | SP | Activity | F, TM |
| The Ambulatory Eye Shield Head Tracking Device with Real-Time Feedback for Gas Filled Eye Patients | Thanawattano, C., et al. | 2019 | Thailand | Feasibility/Validity | Ophthalmology | Post | SP, RGW | Biometrics | F |
| Assessment of Physical Activity by Wearable Technology During Rehabilitation After Cardiac Surgery: Explorative Prospective Monocentric Observational Cohort Study | Thijs, I., et al. | 2019 | Belgium | Original Prospective | Cardiothoracic | Post | CGW | Activity | O |
| Recovery of mandibular motion after closed and open treatment of unilateral mandibular condylar process fractures | Throckmorton, G. S. and E. Ellis | 2000 | USA | Original Prospective | Oromaxillofacial | Post | RGW | Biometrics | TM |
| The monitoring of activity at home after total hip arthroplasty | Toogood, P. A., et al. | 2016 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Normative data of a Smartphone app-based 6-min walking test, test-retest reliability, and content validity with patient-reported outcome measures | Tosic, L., et al. | 2020 | Switzerland | Feasibility/Validity | Neurosurgery | Post | SP | Activity | O |
| Evaluation of improvement in quality of life and physical activity after total knee arthroplasty in greek elderly women | Tsonga, T., et al. | 2011 | Greece | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| Telerehabilitation of Patients with Injuries of the Lower Extremities | Tsvyakh, A. I. and A. J. Hospodarskyy | 2017 | Ukraine | Feasibility/Validity | Orthopaedics | Post | RGW | Activity | O |
| Measurement of physical activity in the Pre- and early Post-operative Period after total knee arthroplasty for Osteoarthritis using a Fitbit Flex device | Twiggs, J., et al. | 2018 | Australia | Original Prospective | Orthopaedics | Pre, Post | CGW | Activity | TM |
| Measuring physical activity in patients after surgery for a malignant tumour in the leg—The reliability and validity of a continuous ambulatory activity monitor | van Dam, M. S., et al. | 2001 | Netherlands | Feasibility/Validity | Surgical Oncology | Post | RGW | Activity | TM |
| Measuring physical activity in patients after surgery for a malignant tumour in the leg. The reliability and validity of a continuous ambulatory activity monitor | van Dam, M. S., et al. | 2001 | Netherlands | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Fatigue, level of everyday physical activity and quality of life after liver transplantation | van den Berg-Emons, R., et al. | 2006 | Netherlands | Original Prospective | Transplant | Post | RGW | Activity | TM |
| Knee kinematics in functional activities seven years after total knee arthroplasty | van der Linden, M. L., et al. | 2006 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Between-day repeatability of knee kinematics during functional tasks recorded using flexible electrogoniometry | van der Linden, M. L., et al. | 2008 | UK | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Exercise therapy after coronary artery bypass graft surgery: a randomized comparison of a high and low frequency exercise therapy program | van der Peijl, I. D., et al. | 2004 | Netherlands | Original Prospective | Cardiothoracic | Post | RGW | Activity | RP |
| Feedback From Activity Trackers Improves Daily Step Count After Knee and Hip Arthroplasty: A Randomized Controlled Trial | Van der Walt, N., et al. | 2018 | Australia | Original Prospective | Orthopaedics | Pre, Post | CGW | Activity | O |
| Validation of a novel activity monitor in impaired, slow-walking, crutch-supported patients | van Laarhoven, S. N., et al. | 2016 | Netherlands | Feasibility/Validity | Orthopaedics | Post | CGW | Activity | F |
| Individual Patient-reported Activity Levels Before and After Joint Arthroplasty Are Neither Accurate nor Reproducible | Vaughn, N. H., et al. | 2019 | USA | Original Prospective | Orthopaedics | Post | CGW | Activity | TM |
| A kinematical analysis of the shoulder after arthroplasty during a hair combing task | Veeger, H. E., et al. | 2006 | Netherlands | Original Prospective | Orthopaedics | Post | RGW | Biometrics | P |
| Grammont versus lateralizing reverse shoulder arthroplasty for proximal humerus fracture: functional and radiographic outcomes | Verdano, M. A., et al. | 2018 | Italy | Original Retrospective | Orthopaedics | Post | RGW | Biometrics | O |
| Walking and chair rising performed in the daily life situation before and after total hip arthroplasty | Vissers, M. M., et al. | 2011 | Netherlands | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | TM |
| Functional capacity and actual daily activity do not contribute to patient satisfaction after total knee arthroplasty | Vissers, M. M., et al. | 2010 | Netherlands | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity | O |
| Function and activity after minimally invasive total hip arthroplasty compared to a healthy population | von Rottkay, E., et al. | 2018 | Germany | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Wearable Sensor-Based Digital Biomarker to Estimate Chest Expansion During Sit-to-Stand Transitions—A Practical Tool to Improve Sternal Precautions in Patients Undergoing Median Sternotomy | Wang, C., et al. | 2019 | USA | Feasibility/Validity | Cardiothoracic | Post | RGW | Biometrics | F |
| Quantifying the influence of DBS surgery in patients with Parkinson’s disease during Perioperative Period by wearable sensors | Wang, J., et al. | 2019 | China | Original Prospective | Neurosurgery | Pre, Peri, Post | RGW | Biometrics | TM |
| Upper extremity function in the free living environment of adults with traumatic brachial plexus injuries | Webber, C. M., et al. | 2019 | USA | Original Prospective | Orthopaedics | Pre, Post | RGW | Activity, Biometrics | TM |
| Sedentary Behavior, Cadence, and Physical Activity Outcomes after Knee Arthroplasty | Webber, S. C., et al. | 2017 | Canada | Original Prospective | Orthopaedics | Post | RGW | Activity | TM, RP |
| Use of Activity Tracking in Major Visceral Surgerythe Enhanced Perioperative Mobilization Trial: a Randomized Controlled Trial | Wolk, S., et al. | 2017 | Germany | Original Prospective | General Surgery | Post | CGW | Activity | F |
| Wearable-Based Mobile Health App in Gastric Cancer Patients for Postoperative Physical Activity Monitoring: Focus Group Study | Wu, J. M., et al. | 2019 | Taiwan | Feasibility/Validity | Surgical Oncology | Pre, Peri, Post | SP | Activity | F |
| Assessing function in patients undergoing joint replacement: a study protocol for a cohort study | Wylde, V., et al. | 2012 | UK | Original Prospective | Orthopaedics | Post | RGW | Activity | TM |
| Implantable Multi-Modality Probe for Subdural Simultaneous Measurement of Electrophysiology, Hemodynamics, and Temperature Distribution | Yamakawa, T., et al. | 2019 | Japan | Feasibility/Validity | Neurosurgery | Peri, Post | RGW | Biometrics | F |
| Sensor-Based Upper-Extremity Frailty Assessment for the Vascular Risk Stratification | Yanquez, F. J., et al. | 2020 | USA | Feasibility/Validity | Vascular | Post | RGW | Biometrics | RP |
| Kinematic study of the temporomandibular joint in normal subjects and patients following unilateral temporomandibular joint arthrotomy with metal fossa-eminence partial joint replacement | Yoon, H. J., et al. | 2007 | South Korea | Original Prospective | Oromaxillofacial | Post | SP, CGW | Biometrics | TM |
| Biomechanical Gait Variable Estimation Using Wearable Sensors after Unilateral Total Knee Arthroplasty | Youn, I. H., et al. | 2018 | South Korea | Feasibility/Validity | Orthopaedics | Post | RGW | Biometrics | F |
| Over-the-top ACL Reconstruction Plus Extra-articular Lateral Tenodesis with Hamstring Tendon Grafts: Prospective Evaluation with 20-Year Minimum Follow-up | Zaffagnini, S., et al. | 2017 | Italy | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
| Assessing activity in joint replacement patients | Zahiri, C. A., et al. | 1998 | USA | Original Prospective | Orthopaedics | Post | Unknown | Activity | TM |
| Evaluation of Gait Variable Change over Time as Transtibial Amputees Adapt to a New Prosthesis Foot | Zhang, X., et al. | 2019 | China | Original Prospective | Orthopaedics | Post | RGW | Biometrics | TM |
Appendix D
| Bariatric Surgery | Obstetrics and Gynecology | Meniscectomy |
| Gastric Bypass Surgery | Cesarian Section | Proximal Femur Fracture Fixation |
| Breast Surgery | Hysterectomy | Proximal Row Carpectomy |
| Mastectomy | Ophthalmologic Surgery | Transtibial Amputation |
| Breast Cancer Surgery | Cataract Surgery | Rotator Cuff Repair |
| Cardiothoracic Surgery | Eye Surgery | Shoulder Surgery |
| Angioplasty | Oromaxillofacial Surgery | Shoulder Arthroplasty |
| Arterial Catheterization | Dental Implantation Surgery | Shoulder Prostheses Surgery |
| Cardiac Surgery | Temporomandibular Joint Replacement | Spinal Stenosis Surgery |
| Coronary Artery Bypass Grafting | Unilateral Mandibular Condylar Fixation | Spine Surgery |
| Elective Cardiac Surgery | Orthopedic Surgery | Total Ankle Arthroplasty |
| Pulmonary Surgery | 3-Corner-Fusion | Total Hip Arthroplasty |
| Lung Cancer Surgery | 4-Corner Fusion | Total Joint Arthroplasty |
| Lung Lobectomy | Achilles Tendon Rupture Repair | Total Knee Arthroplasty |
| Lung Resection | ACL Reconstruction Surgery | Total Wrist Arthroplasty |
| Major Pulmonary Surgery | Ankle Surgery | Vertebroplasty |
| Sternotomy | Back Surgery | Surgical Oncology |
| Thoracic Surgery | Carpal Tunnel Release | Abdominal Cancer Resection |
| Colorectal Surgery | Decompressive Spine Surgery | Major Oncologic Surgery |
| General Surgery | Endoprosthesis Surgery | Pelvic Exenteration |
| Abdominal Surgery | Fracture Repair | Sarcoma Resection |
| Gastric Resection Surgery | Hallux Valgus Correction Surgery | Lower Extremity Tumor Resection |
| Gastrointestinal Resection | Hip Fracture Surgery | Transplant Surgery |
| Hepatic Resection | Hip Surgery | Elective Organ Transplantation |
| Hepatobiliary Resection | Knee Prostheses Surgery | Kidney Transplant Surgery |
| Inguinal Surgery | Limb Salvage Surgery | Liver Transplant Surgery |
| Major Abdominal Surgery | Lower Extremity Orthopedic Surgery | Urologic Surgery |
| Neurosurgery | Lower Limb Amputation Surgery | Cystectomy |
| Brachial Plexus Nerve Transfer Surgery | Lumbar Decompression Surgery | Vascular Surgery |
| Deep Brain Stimulation | Lumbar Microdiscectomy | Lower Limb Amputation Surgery |
| Transsphenoidal Surgery | Lumbar Spine Surgery | |
| Traumatic Brachial Plexus Injury Repair | Malleolar Fracture Fixation |
Appendix E
| Research-Grade Wearables and Sensors | Magnet Sensors (Other) | Activity Tracker/Monitor (Other) | Sportline 345 Pedometer |
| Actigraph AM7164-2.2 activity monitor | Magnetometer (Other) | Apple Watch | Sportline Pedometer |
| Actigraph GT1M accelerometer | Micro-Motion Logger System | Axivity AX3 | SW200 Yamax Digiwalker Pedometer |
| ActiGraph GT3X+ Activity Monitors | Microstrain Inertia Link | BioPACK Tracking Device | USB Accelerometer ModelX8M-3 |
| ActiGraph wGT3X-BT accelerometer | MoLab Portable Motion Sensor System | Digi-Walker SW-200 Pedometer | USB accelerometer X16-mini |
| ActivPAL activity monitor | MTx Inertial Orientation Tracker | Fitbase | Visual Behavior Monitor |
| ADXL 210 acclerometers | MVN Awinda | Fitbit (Other) | Wavelet Health Wristband |
| ADXRS 250 gyroscopes | Noraxon accelerometer | Fitbit Charge | Withings Pulse Ox Activity Monitor |
| AMP-331c Activity Monitor | Nottingham Leg ExtensorPower (LEP) | Fitbit Flex | Yamax FitPro Pedometer |
| Analog Devices accelerometer | Pedar-X | Fitbit Zip | Yamax SW 200/LS2000 Pedometer |
| APDM Movement Monitoring System | POHTRACK (Postoperative Head Tracking Device) | Fitness Tracker (Other) | Smartphone Applications |
| Biometrics XM65 Electrogoniometer | RehaGait R System | Garmin (Other) | 6WT Application |
| BioSensics Triaxial Gyroscope Sensors | Saphon Visi-trainer3 | Garmin Vivoactive HR device | Beiwe Application |
| BioStampRC Sensors | SenseWear Pro2 | Garmin Vivofit2 | Capstesia Application |
| Dynaport ADL monitor | SenseWear Pro3 | GC Dataconcepts LLC Accelerometer Activity Monitor | mHealth Application |
| Electrogoniometer (Other) | SensiumVitals | HITEC Pedometer | Moves Application by Protogeo |
| Exfix Accelerometer | Sensors (other) | Lumo Lift Device | POHTRACK (Postoperative Head Tracking Device) Application |
| Flock of Birds | SG150 Flexible Electrogoniometer | Lumo Run | Rehabilitation Monitoring Application |
| Footswitches | SHIMMER 2R Sensor Units | MetaWear C Sensor Board | Smartphone accelerometer |
| GT9X Link ActiGraph | ShoWIder | MiBand2 | Spine-Specifc 6WT Application |
| GWalk Sensor | Sirognathograph by Siemens Corp | MicrosoftKinect v2 sensor | SurgeryDiary Application |
| Gyroscope (Other) | Sphygmomanometer (Other) | Mio Activity Tracker (Other) | The Motion-Monitor |
| HipGuard | StepWatch 3™Activity Monitor | Misfit Shine | The NeuroPath Application |
| IC-3031 Uniaxial Piezo-resistive Accelerometers | Temec Instruments Accelerometer | New Lifestyles NL-800 Pedometer | The RehabTracker Application |
| Inclination Sensors (Other) | The HealthPatch MD | Omron HJ-321-E Pedometer | TKR Application |
| Inertial Measurement Unit | The PAM | Omron HJ-720 TE2 Pedometer | WalkOn Application |
| Intelligent Device for EnergyExpenditure and Activity | The Wake Forest RTLS | Omron Pedometer | Unknown |
| Kenz Lifecoder GS Accelerometer | Vitaport3 accelerometer | Physilog ®® activity monitor | Pedometer (other) |
| KiRA | Consumer-Grade Wearables | Polar Loop activity tracker | |
| Lifecoder EX Pedometer | 3Space Fastrak System | Power Walker EX-510 Yamax Step Counter | |
| M180 Electrogoniometer | Activ8™ Professional Activity Monitor | Smartwatch (Other) |
References
- Austin, E.; Lee, J.R.; Amtmann, D.; Bloch, R.; Lawrence, S.O.; McCall, D.; Munson, S.; Lavallee, D.C. Use of patient-generated health data across healthcare settings: Implications for health systems. JAMIA Open 2020, 3, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jim, H.; Hoogland, A.I.; Brownstein, N.C.; Barata, A.; Dicker, A.P.; Knoop, H.; Gonzalez, B.D.; Perkins, R.; Rollison, D.; Gilbert, S.M.; et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J. Clin. 2020, 70, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.R.; Kellogg, R.A.; Snyder, M.P.; Dunn, J. Windows into human health through wearables data analytics. Curr. Opin. Biomed. Eng. 2019, 9, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.J.; Grimm, B.; Hanflik, A.M.; Marmor, M.T.; Richter, P.H.; Sands, A.K.; Sivananthan, S. Finding NEEMO: Towards organizing smart digital solutions in orthopaedic trauma surgery. EFORT Open Rev. 2020, 5, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Krummel, T. The digital surgeon: How big data, automation, and artificial intelligence will change surgical practice. J. Pediatr. Surg. 2020, 55, 47–50. [Google Scholar] [CrossRef]
- Jain, S.H.; Powers, B.W.; Hawkins, J.B.; Brownstein, J.S. The digital phenotype. Nat. Biotechnol. 2015, 33, 462–463. [Google Scholar] [CrossRef]
- Insel, T.R. Digital phenotyping: Technology for a new science of behavior. JAMA 2017, 318, 1215–1216. [Google Scholar] [CrossRef]
- Vaidyam, A.; Halamka, J.; Torous, J. Actionable digital phenotyping: A framework for the delivery of just-in-time and longitudinal interventions in clinical healthcare. Mhealth 2019, 5, 25. [Google Scholar] [CrossRef]
- Huckvale, K.; Venkatesh, S.; Christensen, H. Toward clinical digital phenotyping: A timely opportunity to consider purpose, quality, and safety. NPJ Digit. Med. 2019, 2, 88. [Google Scholar] [CrossRef] [Green Version]
- Barnett, S.; Huckvale, K.; Christensen, H.; Venkatesh, S.; Mouzakis, K.; Vasa, R. Intelligent Sensing to Inform and Learn (InSTIL): A scalable and governance-aware platform for universal, smartphone-based digital phenotyping for research and clinical applications. J. Med. Internet Res. 2019, 21, e16399. [Google Scholar] [CrossRef]
- Chang, C.-H. Patient-Reported outcomes measurement and management with innovative methodologies and technologies. Qual. Life Res. 2007, 16, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Black, N. Patient reported outcome measures could help transform healthcare. BMJ 2013, 346, f167. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, J.; Dalkin, S.; Gooding, K.; Gibbons, E.; Wright, J.; Meads, D.; Black, N.; Valderas, J.M.; Pawson, R. Functionality and feedback: A realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Serv. Deliv. Res. 2017, 5, 1–280. [Google Scholar] [CrossRef] [PubMed]
- Muehlhausen, W.; Doll, H.; Quadri, N.; Fordham, B.; O’Donohoe, P.; Dogar, N.; Wild, D.J. Equivalence of electronic and paper administration of patient-reported outcome measures: A systematic review and meta-analysis of studies conducted between 2007 and 2013. Health Qual. Life Outcomes 2015, 13, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.E.; Rothrock, N.E.; DeWitt, E.M.; Spiegel, B.; Tucker, C.A.; Crane, H.M.; Forrest, C.B.; Patrick, D.L.; Fredericksen, R.; Shulman, L.M.; et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med. Care 2015, 53, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Black, N.; Varaganum, M.; Hutchings, A. Relationship between patient reported experience (PREMs) and patient reported outcomes (PROMs) in elective surgery. BMJ Qual. Saf. 2014, 23, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Butow, P.; Dhillon, H.; Sundaresan, P. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J. Med. Radiat. Sci. 2020. [Google Scholar] [CrossRef]
- Panda, N.; Solsky, I.; Huang, E.J.; Lipsitz, S.; Pradarelli, J.C.; Delisle, M.; Cusack, J.C.; Gadd, M.A.; Lubitz, C.C.; Mullen, J.T.; et al. Using smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg. 2020, 155, 123–129. [Google Scholar] [CrossRef]
- Khangura, S.; Konnyu, K.; Cushman, R.; Grimshaw, J.; Moher, D. Evidence summaries: The evolution of a rapid review approach. Syst. Rev. 2012, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO|Rapid Reviews to Strengthen Health Policy and Systems: A Practical Guide. Available online: http://www.who.int/alliance-hpsr/resources/publications/rapid-review-guide/en/ (accessed on 21 September 2020).
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Shantz, J.A.S.; Veillette, C.J.H. The application of wearable technology in surgery: Ensuring the positive impact of the wearable revolution on surgical patients. Front. Surg. 2014, 1, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, R.; van Limbeek, J.; Kriek, B.; Ihnenfeldt, D.; Vermeulen, M.; de Haan, R. Prognostic social factors in the subacute phase after a stroke for the discharge destination from the hospital stroke-unit. A systematic review of the literature. Disabil. Rehabil. 2004, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kwasnicki, R.M.; Ali, R.; Jordan, S.J.; Atallah, L.; Leong, J.J.; Jones, G.G.; Cobb, J.; Yang, G.Z.; Darzi, A. A wearable mobility assessment device for total knee replacement: A longitudinal feasibility study. Int. J. Surg. 2015, 18, 14–20. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chen, K.-H.; Liu, K.-C.; Hsu, S.J.-P.; Chan, C.-T. Data collection and analysis using wearable sensors for monitoring knee range of motion after total knee arthroplasty. Sensors 2017, 17, 418. [Google Scholar] [CrossRef]
- Youn, I.-H.; Youn, J.-H.; Zeni, J.A.; Knarr, B.A. Biomechanical gait variable estimation using wearable sensors after unilateral total knee arthroplasty. Sensors 2018, 18, 1577. [Google Scholar] [CrossRef] [Green Version]
- Teufl, W.; Taetz, B.; Miezal, M.; Lorenz, M.; Pietschmann, J.; Jöllenbeck, T.; Fröhlich, M.; Bleser, G. Towards an inertial sensor-based wearable feedback system for patients after total hip arthroplasty: Validity and applicability for gait classification with gait kinematics-based features. Sensors 2019, 19, 5006. [Google Scholar] [CrossRef] [Green Version]
- Cote, D.J.; Barnett, I.; Onnela, J.-P.; Smith, T.R. Digital phenotyping in patients with spine disease: A novel approach to quantifying mobility and quality of life. World Neurosurg. 2019. [Google Scholar] [CrossRef]
- Buchman, A.S.; Dawe, R.J.; Leurgans, S.E.; Curran, T.A.; Truty, T.; Yu, L.; Barnes, L.L.; Hausdorff, J.M.; Bennett, D.A. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J. Gerontol. Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Montag, C.; Sindermann, C.; Baumeister, H. Digital phenotyping in psychological and medical sciences: A reflection about necessary prerequisites to reduce harm and increase benefits. Curr. Opin. Psychol. 2020, 36, 19–24. [Google Scholar] [CrossRef]
- Cohen, A.B.; Mathews, S.C. The digital outcome measure. Digit. Biomark. 2018, 2, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Priemer, U.; Santangelo, P. Digital phenotyping: Hype or hope? Lancet Psychiatry 2020, 7, 297–299. [Google Scholar] [CrossRef]
- Marsch, L.A. Digital health data-driven approaches to understand human behavior. Neuropsychopharmacology 2021. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, N.; Char, D. Surveillance and digital health. Am. J. Bioeth. 2018, 18, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Onnela, J.-P.; Rauch, S.L. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology 2016, 41, 1691–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, N.C.; Summers, B.; Wilhelm, S. Digital biomarkers of social anxiety severity: Digital phenotyping using passive smartphone sensors. J. Med. Internet Res. 2020, 22, e16875. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.C.; Weingarden, H.; Wilhelm, S. Using digital phenotyping to accurately detect depression severity. J. Nerv. Ment. Dis. 2019, 207, 893–896. [Google Scholar] [CrossRef]
- Raballo, A. Digital phenotyping: An overarching framework to capture our extended mental states. Lancet Psychiatry 2018, 5, 194–195. [Google Scholar] [CrossRef]
- Torous, J.; Staples, P.; Barnett, I.; Onnela, J.-P.; Keshavan, M. A crossroad for validating digital tools in schizophrenia and mental health. NPJ Schizophr. 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, E.M.; Turner, B.J.; Fedor, S.; Beale, E.E.; Picard, R.W.; Huffman, J.C.; Nock, M.K. Digital phenotyping of suicidal thoughts. Depress. Anxiety 2018, 35, 601–608. [Google Scholar] [CrossRef]
- Moukaddam, N.; Truong, A.; Cao, J.; Shah, A.; Sabharwal, A. Findings from a Trial of the Smartphone and OnLine Usage-based eValuation for Depression (SOLVD) application: What do apps really tell us about patients with depression? Concordance between app-generated data and standard psychiatric questionnaires for depression and anxiety. J. Psychiatr. Pract. 2019, 25, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Guimond, S.; Keshavan, M.S.; Torous, J.B. Towards remote digital phenotyping of cognition in schizophrenia. Schizophr. Res. 2019, 208, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, J.; Piscitello, A.; Rasic, M.; Easter, R.; Babu, P.; Langenecker, S.A.; McInnis, M.G.; Ajilore, O.; Nelson, P.C.; Ryan, K.A.; et al. Predicting mood disturbance severity with mobile phone keystroke metadata: A biaffect digital phenotyping study. J. Med. Internet Res. 2018, 20, e241. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.; Henson, P.; Torous, J. Using a smartphone app to identify clinically relevant behavior trends via symptom report, cognition scores, and exercise levels: A case series. Front. Psychiatry 2019, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.D.; Paganoni, S.; Carlson, K.; Burke, K.; Weber, H.; Staples, P.; Salinas, J.; Chan, J.; Green, J.R.; Connaghan, K.; et al. Design and results of a smartphone-based digital phenotyping study to quantify ALS progression. Ann. Clin. Transl. Neurol. 2019, 6, 873–881. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/ wearable devices opportunity. NPJ Digit. Med. 2019, 2. [Google Scholar] [CrossRef]
- Doryab, A.; Villalba, D.K.; Chikersal, P.; Dutcher, J.M.; Tumminia, M.; Liu, X.; Cohen, S.; Creswell, K.G.; Mankoff, J.; Creswell, J.D.; et al. Identifying behavioral phenotypes of loneliness and social isolation with passive sensing: Statistical analysis, data mining and machine learning of smartphone and fitbit data. JMIR Mhealth Uhealth 2019, 7, e13209. [Google Scholar] [CrossRef] [Green Version]
- Skinner, A.L.; Attwood, A.S.; Baddeley, R.; Evans-Reeves, K.; Bauld, L.; Munafò, M.R. Digital phenotyping and the development and delivery of health guidelines and behaviour change interventions. Addiction 2017, 112, 1281–1285. [Google Scholar] [CrossRef] [Green Version]
- Papi, E.; Koh, W.S.; McGregor, A.H. Wearable technology for spine movement assessment: A systematic review. J. Biomech. 2017, 64, 186–197. [Google Scholar] [CrossRef]
- Papi, E.; Belsi, A.; McGregor, A.H. A knee monitoring device and the preferences of patients living with osteoarthritis: A qualitative study. BMJ Open 2015, 5, e007980. [Google Scholar] [CrossRef] [Green Version]
- Papi, E.; Osei-Kuffour, D.; Chen, Y.-M.A.; McGregor, A.H. Use of wearable technology for performance assessment: A validation study. Med. Eng. Phys. 2015, 37, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breteler, M.J.M.; Kleinjan, E.; Numan, L.; Ruurda, J.P.; Van Hillegersberg, R.; Leenen, L.P.; Hermans, M.C.; Kalkman, C.J.; Blokhuis, T.J. Are current wireless monitoring systems capable of detecting adverse events in high-risk surgical patients? A descriptive study. Injury 2020, 51, S97–S105. [Google Scholar] [CrossRef] [PubMed]
- Maher, N.A.; Senders, J.T.; Hulsbergen, A.F.; Lamba, N.; Parker, M.; Onnela, J.-P.; Bredenoord, A.L.; Smith, T.R.; Broekman, M.L.D. Passive data collection and use in healthcare: A systematic review of ethical issues. Int. J. Med. Inform. 2019, 129, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Warraich, H.J.; Califf, R.M.; Krumholz, H.M. The digital transformation of medicine can revitalize the patient-clinician relationship. NPJ Digit. Med. 2018, 1, 49. [Google Scholar] [CrossRef] [Green Version]
- Rieger, A.; Gaines, A.; Barnett, I.; Baldassano, C.F.; Gibbons, M.B.C.; Crits-Christoph, P. Psychiatry outpatients’ willingness to share social media posts and smartphone data for research and clinical purposes: Survey study. JMIR Form. Res. 2019, 3, e14329. [Google Scholar] [CrossRef]
- Pevnick, J.M.; Fuller, G.; Duncan, R.; Spiegel, B.M.R. A large-scale initiative inviting patients to share personal fitness tracker data with their providers: Initial results. PLoS ONE 2016, 11, e0165908. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Gooding, K.; Gibbons, E.; Dalkin, S.; Wright, J.; Valderas, J.M.; Black, N. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J. Patient Rep. Outcomes 2018, 2, 42. [Google Scholar] [CrossRef]
- Buchlak, Q.D.; Esmaili, N.; Leveque, J.-C.; Farrokhi, F.; Bennett, C.; Piccardi, M.; Sethi, R.K. Machine learning applications to clinical decision support in neurosurgery: An artificial intelligence augmented systematic review. Neurosurg. Rev. 2020, 43, 1235–1253. [Google Scholar] [CrossRef] [Green Version]
- Higaki, A.; Uetani, T.; Ikeda, S.; Yamaguchi, O. Co-Authorship network analysis in cardiovascular research utilizing machine learning (2009–2019). Int. J. Med. Inform. 2020, 143. [Google Scholar] [CrossRef]







| Category | Number of Publications | |
|---|---|---|
| Country | USA | 74 |
| UK | 23 | |
| Netherlands | 21 | |
| Australia | 17 | |
| Germany | 14 | |
| Switzerland | 10 | |
| Canada | 7 | |
| Spain | 7 | |
| Japan | 6 | |
| Italy | 5 | |
| South Korea | 5 | |
| Belgium | 4 | |
| Norway | 4 | |
| Sweden | 4 | |
| Brazil | 3 | |
| Denmark | 3 | |
| Taiwan | 3 | |
| China | 2 | |
| Greece | 2 | |
| Finland | 1 | |
| France | 1 | |
| Israel | 1 | |
| Portugal | 1 | |
| Romania | 1 | |
| Serbia | 1 | |
| South Africa | 1 | |
| Thailand | 1 | |
| Turkey | 1 | |
| Ukraine | 1 | |
| Surgical Specialty | Bariatric | 10 |
| Breast | 1 | |
| Cardiothoracic | 15 | |
| Colorectal | 2 | |
| General | 13 | |
| Neurosurgery | 24 | |
| Obstetrics/Gynecology | 2 | |
| Ophthalmology | 3 | |
| Oromaxillofacial | 6 | |
| Orthopaedics | 129 | |
| Surgical Oncology | 11 | |
| Transplant | 7 | |
| Urologic | 1 | |
| Vascular | 4 | |
| Pathway Phase | Post | 171 |
| Peri | 4 | |
| Pre, Post | 36 | |
| Pre, Peri, Post | 2 | |
| Peri, Post | 1 | |
| Pre | 10 | |
| Data Type | Activity | 122 |
| Biometrics | 59 | |
| Communication | 0 | |
| Activity, Biometrics | 41 | |
| Activity, Communication | 2 | |
| Function | Feasibility | 61 |
| Tracking or Monitoring | 82 | |
| Prediction | 18 | |
| Risk Profiling | 8 | |
| Optimization | 18 | |
| Feasibility, Tracking or Monitoring | 10 | |
| Feasibility, Prediction | 1 | |
| Feasibility, Risk Profiling, Prediction | 1 | |
| Feasibility, Tracking or Monitoring, Prediction | 3 | |
| Feasibility, Tracking or Monitoring, Risk Profiling | 2 | |
| Risk Profiling, Prediction | 1 | |
| Risk Profiling, Prediction, Optimization | 1 | |
| Tracking or Monitoring, Optimization | 5 | |
| Tracking or Monitoring, Prediction | 6 | |
| Tracking or Monitoring, Risk Profiling | 5 | |
| Tracking or Monitoring, Risk Profiling, Optimization | 1 | |
| Tracking or Monitoring, Risk Profiling, Prediction | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakumar, P.; Lin, E.; Galea, V.; Mathew, A.J.; Panda, N.; Vetter, I.; Haynes, A.B. Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review. J. Pers. Med. 2020, 10, 282. https://doi.org/10.3390/jpm10040282
Jayakumar P, Lin E, Galea V, Mathew AJ, Panda N, Vetter I, Haynes AB. Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review. Journal of Personalized Medicine. 2020; 10(4):282. https://doi.org/10.3390/jpm10040282
Chicago/Turabian StyleJayakumar, Prakash, Eugenia Lin, Vincent Galea, Abraham J. Mathew, Nikhil Panda, Imelda Vetter, and Alex B. Haynes. 2020. "Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review" Journal of Personalized Medicine 10, no. 4: 282. https://doi.org/10.3390/jpm10040282
APA StyleJayakumar, P., Lin, E., Galea, V., Mathew, A. J., Panda, N., Vetter, I., & Haynes, A. B. (2020). Digital Phenotyping and Patient-Generated Health Data for Outcome Measurement in Surgical Care: A Scoping Review. Journal of Personalized Medicine, 10(4), 282. https://doi.org/10.3390/jpm10040282