Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder
Abstract
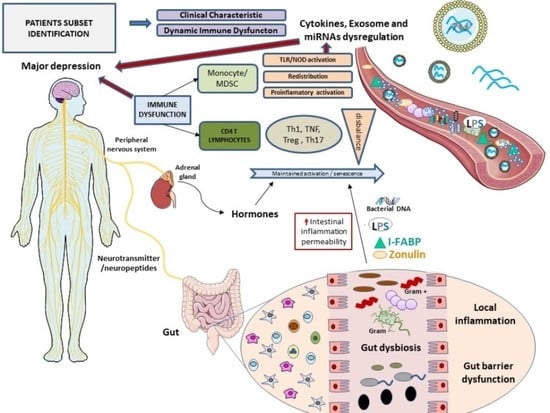
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Isolation of Peripheral Blood Mononuclear Cells
2.3. Surface CD28 Lymphocyte Staining
2.4. In Vitro Culture
2.5. Intracellular Lymphocyte Cytokines Assay
2.6. Cytokines Serum Levels
2.7. Statistical Analysis
3. Results
3.1. Patient Demographic Characteristics
3.2. MDD Patients Show Increased Counts of the TE CD4 T Lymphocyte Subset
3.3. MDD Patients Show an Expansion of Circulating Th17 and TNF+ CD4+ T Lymphocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Malhi, G.S.; Mann, J.J. Depression. Lancet. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673618319482 (accessed on 24 November 2018).
- Niemegeers, P.; De Boer, P.; Schuermans, J.; Dumont, G.J.; Coppens, V.; Spittaels, K.; Claes, S.; Sabbe, B.G.; Morrens, M. Digging deeper in the differential effects of inflammatory and psychosocial stressors in remitted depression: Effects on cognitive functioning. J. Affect. Disord. 2019, 245, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27752078 (accessed on 17 November 2020). [CrossRef] [Green Version]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H.; Takiguchi, M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10537110 (accessed on 17 November 2020). [CrossRef]
- Sallusto, F.; Monticelli, S. The many faces of CD4 T cells: Roles in immunity and disease. Semin. Immunol. 2013, 25, 249–251. [Google Scholar] [CrossRef]
- Schmitt, N.; Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 2015, 34, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahedi, G.; Takahashi, H.; Nakayamada, S.; Sun, H.-W.; Sartorelli, V.; Kanno, Y.; O’Shea, J.J. STATs Shape the Active Enhancer Landscape of T Cell Populations. Cell 2012, 151, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R.B. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15032595 (accessed on 17 November 2020). [CrossRef]
- Taylor, J.J.; Jenkins, M.K. CD4+ memory T cell survival. Curr. Opin. Immunol. 2011, 23, 319–323. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Bin Wei, Y.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R.; et al. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry 2019, 25, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, R.; Kumar, P.K.; Mitra, P.; Purohit, P.; Nebhinani, N.; Sharma, P. Circulating T helper 17 and IFN-γ positive Th17 cells in Major Depressive Disorder. Behav. Brain Res. 2020, 394, 112811. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9881538 (accessed on 30 October 2019).
- Alvarez-Mon, M.A.; Gómez, A.M.; Orozco, A.; Lahera, G.; Sosa, M.D.; Diaz, D.; Auba, E.; Albillos, A.; Monserrat, J.; Alvarez-Mon, M. Abnormal Distribution and Function of Circulating Monocytes and Enhanced Bacterial Translocation in Major Depressive Disorder. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Snijders, G.; Schiweck, C.; Mesman, E.; Grosse, L.; De Wit, H.; Nolen, W.A.; Drexhage, H.A.; Hillegers, M.H.J. A dynamic course of T cell defects in individuals at risk for mood disorders. Brain Behav. Immun. 2016, 58, 11–17. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, Y.-K.; Hwang, J.-A.; Yoon, H.-K.; Ko, Y.-H.; Han, C.; Lee, H.-J.; Ham, B.-J.; Lee, H.-S. Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig. 2013, 10, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davami, M.H.; Baharlou, R.; Vasmehjani, A.A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M.R. Elevated IL-17 and TGF-β Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. J. 2016, 7, 137–142. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Luborsky, L.; McKay, J.R.; Rosenthal, R.; Houldin, A.; Tax, A.; McCorkle, R.; Seligman, D.A.; Schmidt, K. The Relationship of Depression and Stressors to Immunological Assays: A Meta-Analytic Review. Brain Behav. Immun. 2001, 15, 199–226. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11566046 (accessed on 19 November 2020). [CrossRef] [Green Version]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, H.; Yao, W.; Bian, F.; Mao, X.; Yang, X.; Jin, S. Antidepressant Drug, Desipramine, Alleviates Allergic Rhinitis by Regulating Treg and Th 17 Cells. Int. J. Immunopathol. Pharmacol. 2013, 26, 107–115. [Google Scholar] [CrossRef]
- Schiweck, C.; Valles-Colomer, M.; Arolt, V.; Müller, N.; Raes, J.; Wijkhuijs, A.; Claes, S.; Drexhage, H.; Vrieze, E. Depression and suicidality: A link to premature T helper cell aging and increased Th17 cells. Brain, Behav. Immun. 2020, 87, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Gomez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Sosa-Reina, M.D.; Diaz, D.; Albillos, A.; Quintero, J.; Molero, P.; Monserrat, J.; et al. Blunted Expansion of Regulatory T Lymphocytes Is Associated With Increased Bacterial Translocation in Patients With Major Depressive Disorder. Front. Psychiatry 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Accogli, T.; Bruchard, M.; Végran, F. Modulation of CD4 T Cell Response According to Tumor Cytokine Microenvironment. Cancers 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Köhler, C.A.; Anderson, G.; Quevedo, J.; Alves, G.S.; Berk, M.; Fernandes, B.S.; Carvalho, A.F. T helper 17 cells may drive neuroprogression in major depressive disorder: Proposal of an integrative model. Neurosci. Biobehav. Rev. 2016, 64, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Becking, K.; Haarman, B.C.; Grosse, L.; Nolen, W.A.; Claes, S.; Arolt, V.; Schoevers, R.A.; Drexhage, H.A. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J. Neuroimmunol. 2018, 319, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Köhler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014, 71, 1381–1391. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25322082 (accessed on 17 November 2020). [CrossRef]
- Lee, Y.; Subramaniapillai, M.; Brietzke, E.; Mansur, R.B.; Ho, R.C.; Yim, S.J.; McIntyre, R.S. Anti-cytokine agents for anhedonia: Targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther. Adv. Psychopharmacol. 2018, 8, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22945416 (accessed on 17 November 2020). [CrossRef]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.L.; Mayes, T.L.; Trivedi, M.H. Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav. Immun. 2017, 66, 103–110. [Google Scholar] [CrossRef]
- Hennings, J.M.; Uhr, M.; Klengel, T.; Weber, P.; Pütz, B.; Touma, C.; Czamara, D.; Ising, M.; Holsboer, F.; Lucae, S. RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response. Transl. Psychiatry 2015, 5, e538. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25826113 (accessed on 17 November 2020). [CrossRef] [PubMed] [Green Version]
- Monserrat, J.; Bohórquez, C.; Lahoz, A.M.G.; Movasat, A.; Pérez, A.; Ruíz, L.; Díaz, D.; Chara, L.; Sánchez, A.I.; Albarrán, F.; et al. The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells 2019, 8, 871. [Google Scholar] [CrossRef] [Green Version]
- Weyand, C.M.; Yang, Z.; Goronzy, J.J. T-cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 2014, 26, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, R.; Liu, L.; Qiao, D.; Baldwin, D.S.; Hou, R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2019, 79, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Martinez-Fong, D.; Perez-Tapia, M.; Estrada-Garcia, I.; Estrada-Parra, S.; Pavón, L. Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur. Neuropsychopharmacol. 2010, 20, 88–95. [Google Scholar] [CrossRef] [PubMed]





| MDD | HC | p Value | |
|---|---|---|---|
| Socio-demographic | |||
| Age, mean (SD) | 43.26 (13.14) | 41.45 (11.46) | 0.35 |
| Sex (% female) | 19 (63.3%) | 21 (70%) | 0.98 |
| Currently employed and active n (%) | 13 (59.1%) | 27 (90%) | <0.01 |
| College degree n (%) | 16 (53.3%) | 20 (66.6%) | 0.45 |
| Past History | |||
| Family history of depression n (%) | 17 (56.7%) | 12 (40%) | 0.38 |
| Family history of other psychiatric disorder n (%) | 22 (77.3%) | 14 (46.6%) | 0.22 |
| Health characteristics and somatic comorbidities | |||
| BMI, mean (SD) | 26.74 (5.41) | 25.5 (5.36) | 0.51 |
| Smoking n (%) | 0.13 | ||
| - Never | 12 (40%) | 8 (26.6%) | |
| - Occasionally | 8 (26.7%) | 15 (50%) | |
| - Everyday | 10 (33.3%) | 7 (23.3%) | |
| Drinking n (%) | 0.3 | ||
| - Never | 7 (23.3%) | 6 (20%) | |
| - Occasionally | 20 (66.7%) | 22 (73.3%) | |
| - Everyday | 3 (10%) | 2 (6.6%) | |
| HC (%) | MDD (%) | HC (Cells/L) | MDD (Cells/L) | |
|---|---|---|---|---|
| Th | 75.22 ± 5.77 | 76.45 ± 2.44 | 1038.99 ± 222.94 | 1037.98 ± 88.55 |
| T N | 60.06 ± 6.94 | 62.31 ± 2.88 | 693.05 ± 191.81 | 621.58 ± 51.74 |
| T CM | 32.37 ± 6.12 | 29.29 ± 2.31 | 292.05 ± 77.33 | 326.43 ± 51.12 |
| T EM | 6.64 ± 1.27 | 7.01 ± 0.79 | 48.70 ± 8.95 | 76.15 ± 11.75 |
| T E | 0.91 ± 0.39 | 1.41 ± 0.34* | 5.13 ± 1.67 | 14.06 ± 2.72 * |
| HC (%) | MDD (%) | HC (Cells/L) | MDD (Cells/L) | |
|---|---|---|---|---|
| Th CD28- | 13.66 ± 4.78 | 13.52 ± 2.63 | 149.38 ± 62.90 | 129.57 ± 30.02 |
| T N CD28- | 10.63 ± 3.28 | 9.96 ± 1.81 | 232.05 ± 79.57 | 244.89 ± 38.32 |
| T CM CD28- | 14.43 ± 6.55 | 5.04 ± 1.05 | 25.05 ± 9.99 | 16.97 ± 5.00 |
| T EM CD28- | 24.87 ± 10.63 | 16.61 ± 3.10 | 7.85 ± 3.14 | 14.12 ± 3.49 |
| T E CD28- | 37.27 ± 11.45 | 48.91 ± 6.70* | 2.74 ± 1.49 | 8.78 ± 2.56 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.A.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. https://doi.org/10.3390/jpm11030220
Alvarez-Mon MA, Gómez-Lahoz AM, Orozco A, Lahera G, Diaz D, Ortega MA, Albillos A, Quintero J, Aubá E, Monserrat J, et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. Journal of Personalized Medicine. 2021; 11(3):220. https://doi.org/10.3390/jpm11030220
Chicago/Turabian StyleAlvarez-Mon, Miguel Angel, Ana Maria Gómez-Lahoz, Arancha Orozco, Guillermo Lahera, David Diaz, Miguel A. Ortega, Agustin Albillos, Javier Quintero, Enrique Aubá, Jorge Monserrat, and et al. 2021. "Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder" Journal of Personalized Medicine 11, no. 3: 220. https://doi.org/10.3390/jpm11030220
APA StyleAlvarez-Mon, M. A., Gómez-Lahoz, A. M., Orozco, A., Lahera, G., Diaz, D., Ortega, M. A., Albillos, A., Quintero, J., Aubá, E., Monserrat, J., & Alvarez-Mon, M. (2021). Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. Journal of Personalized Medicine, 11(3), 220. https://doi.org/10.3390/jpm11030220