3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential
Abstract
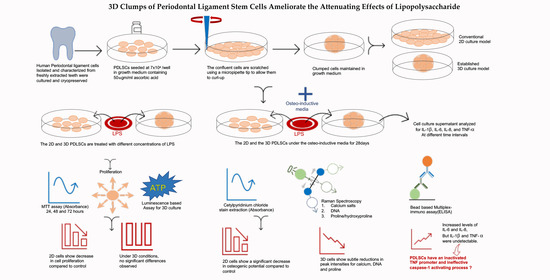
:1. Introduction
2. Materials and Methods
2.1. Cell Proliferation Studies
2.2. Osteogenic Induction
2.3. Prearation of Clumps of Periodontal MSCs (C-MSCs)
2.4. ATP-Based Cell Proliferation Assay for C-MSCs
2.5. Osteogenic Induction of Clumps of MSCs
2.6. Raman Spectroscopic Analysis
2.7. Multiplex Cytokine Immunoassay
2.8. Statistical Analysis
3. Results
3.1. MTT Assay
3.2. Alizarin Red Quantification
3.3. Creation of C-MSCs
3.4. ATP-Based Cell Proliferation Assay
3.5. Quantification of Osteogenic Differentiation of C-MSCs
3.6. Multiplex Immunoassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ATP | Adenosine triphosphate |
| C-MSC | Clumps of mesenchymal stem cell |
| CD | Cluster of differentiation |
| CCM | Complete culture media |
| CPC | Cetylpyridinium chloride |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DNA | Deoxyribonucleic acid |
| ECM | Extra cellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| MIP | Macrophage inflammatory protein |
| MSC | Mesenchymal Stem cell |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-dophenyltetrazolium bromide |
| NF-kB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| OP | Osteogenic potential |
| P | Passage |
| PCA | Principal component analysis |
| PDL | Periodontal ligament |
| PDLSC | Periodontal ligament mesenchymal stem cell |
| RNA | Ribonucleic acid |
| RT | Room temperature |
| SCAPS | Stem cells from the apical papilla |
| SPSS | Statistical Package for Social Sciences, TLR—Toll-like receptors |
| Th | T helper |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
References
- Zhu, W.; Liang, M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.C.; Moreira, A.F.; De Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; A Kunz-Schughart, L. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shoemaker, J.T.; Zhang, W.; Atlas, S.I.; Bryan, R.A.; Inman, S.W.; Vukasinovic, J. A 3D Cell Culture Organ-on-a-Chip Platform With a Breathable Hemoglobin Analogue Augments and Extends Primary Human Hepatocyte Functions in vitro. Front. Mol. Biosci. 2020, 7, 568777. [Google Scholar] [CrossRef]
- Baert, Y.; Rombaut, C.; Goossens, E. Scaffold-Based and Scaffold-Free Testicular Organoids from Primary Human Testicular Cells. Methods Mol. Biol. 2019, 1576, 283–290. [Google Scholar]
- Kittaka, M.; Kajiya, M.; Shiba, H.; Takewaki, M.; Takeshita, K.; Khung, R.; Fujita, T.; Iwata, T.; Nguyen, T.Q.; Ouhara, K.; et al. Clumps of a mesenchymal stromal cell/extracellular matrix complex can be a novel tissue engineering therapy for bone regeneration. Cytotherapy 2015, 17, 860–873. [Google Scholar] [CrossRef]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T.; et al. MSC/ECM Cellular Complexes Induce Periodontal Tissue Regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Usui, M.; Moritani, Y.; Nakazawa, K.; Hanatani, T.; Kondo, H.; Nakatomi, M.; Onizuka, S.; Iwata, T.; Sato, T.; et al. Co-cultured spheroids of human periodontal ligament mesenchymal stem cells and vascular endothelial cells enhance periodontal tissue regeneration. Regen. Ther. 2020, 14, 59–71. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.-Y.; Zhou, H.; Zhou, G.-W. Characterization of mesenchymal stem cells under the stimulation of Toll-like receptor agonists. Dev. Growth Differ. 2014, 56, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.S.; Liu, L.Y.; Chai, J.K.; Yu, Y.H.; Duan, H.J.; Hui, Y.Y.; Ying, L.L.; Wang, Y.H.; Zhuang, S.B.; Fan, J.; et al. Lipopolysaccharide pretreatment inhibits LPS-induced human umbilical cord mesenchymal stem cell apoptosis via upregulating the expression of cellular FLICE-inhibitory protein. Mol. Med. Rep. 2015, 12, 2521–2528. [Google Scholar] [CrossRef]
- Hui, Q.; Fan, S.F.; Feng, Z.Q.; Wang, F.F.; Hou, X.W. The inhibitory effect of mesenchymal stem cell conditioned medium on the secretion of inflammatory factor of human gingival fibroblast induced by lipopolysaccharide. Int. J. Clin. Exp. Med. 2018, 11, 5715–5721. [Google Scholar]
- Herzmann, N.; Salamon, A.; Fiedler, T.; Peters, K. Lipopolysaccharide induces proliferation and osteogenic differentiation of adipose-derived mesenchymal stromal cells in vitro via TLR4 activation. Exp. Cell Res. 2017, 350, 115–122. [Google Scholar] [CrossRef]
- Kooshki, H.; Ghollasi, M.; Halabian, R.; Kazemi, N.M. Osteogenic differentiation of preconditioned bone marrow mesenchymal stem cells with lipopolysaccharide on modified poly-l-lactic-acid nanofibers. J. Cell. Physiol. 2019, 234, 5343–5353. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Singhrao, S.K. Importance of heterogeneity inPorhyromonas gingivalislipopolysaccharide lipid A in tissue specific inflammatory signalling. J. Oral Microbiol. 2018, 10, 1440128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behm, C.; Blufstein, A.; Abhari, S.Y.; Koch, C.; Gahn, J.; Schäffer, C.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Response of Human Mesenchymal Stromal Cells from Periodontal Tissue to LPS Depends on the Purity but Not on the LPS Source. Mediat. Inflamm. 2020, 2020, 8704896. [Google Scholar] [CrossRef] [PubMed]
- Banavar, S.R.; Rawal, S.Y.; Paterson, I.C.; Singh, G.; Davamani, F.; Khoo, S.P.; Tan, E.L.; Amalraj, F.D. Establishing a technique for isolation and characterization of human periodontal ligament derived mesenchymal stem cells. Saudi Dent. J. 2020. [Google Scholar] [CrossRef]
- Keong, J.Y.; Low, L.W.; Chong, J.M.; Ong, Y.Y.; Pulikkotil, S.J.; Singh, G.; Nagendrababu, V.; Banavar, S.R.; Khoo, S.P. Effect of lipopolysaccharide on cell proliferation and vascular endothelial growth factor secretion of periodontal ligament stem cells. Saudi Dent. J. 2020, 32, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Nakata, K.; Imaizumi, I.; Iwama, A.; Nakane, A.; Nakamura, H. Cytotoxic effect of endodontic bacteria on periapical fibroblasts. J. Endod. 1998, 24, 534–539. [Google Scholar] [CrossRef]
- Lertchirakarn, V.; Aguilar, P. Effects of Lipopolysaccharide on the Proliferation and Osteogenic Differentiation of Stem Cells from the Apical Papilla. J. Endod. 2017, 43, 1835–1840. [Google Scholar] [CrossRef]
- Andrukhov, O.; Andrukhova, O.; Özdemir, B.; Haririan, H.; Müller-Kern, M.; Moritz, A.; Rausch-Fan, X. Soluble CD14 Enhances the Response of Periodontal Ligament Stem Cells to P. gingivalis Lipopolysaccharide. PLoS ONE 2016, 11, e0160848. [Google Scholar] [CrossRef]
- Kurte, M.; Vega-Letter, A.M.; Luz-Crawford, P.; Djouad, F.; Noël, D.; Khoury, M.; Carrión, F. Time-dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAE. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, H.-S.; Hong, I.-S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef] [PubMed]
- Motoike, S.; Kajiya, M.; Komatsu, N.; Horikoshi, S.; Ogawa, T.; Sone, H.; Matsuda, S.; Ouhara, K.; Iwata, T.; Mizuno, N.; et al. Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis. Int. J. Mol. Sci. 2019, 20, 3970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.-M.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Kwon, S.-Y.; Lee, H.-S.; Park, J.-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Cohly, H.; Stephens, J.; Markhov, A.; Angel, M.; Campbell, W.; Ndebele, K.; Jenkins, J.; Cohly, H. Cell culture conditions affect lps inducibility of the inflammatory mediators in j774a.1 murine macrophages. Immunol. Investig. 2001, 30, 1–15. [Google Scholar] [CrossRef]
- Short, K.W.; Carpenter, S.; Freyer, J.P.; Mourant, J.R. Raman Spectroscopy Detects Biochemical Changes Due to Proliferation in Mammalian Cell Cultures. Biophys. J. 2005, 88, 4274–4288. [Google Scholar] [CrossRef] [Green Version]
- Omberg, K.M.; Osborn, J.C.; Zhang, S.L.; Freyer, J.P.; Mourant, J.R.; Schoonover, J.R. Raman Spectroscopy and Factor Analysis of Tumorigenic and Non-Tumorigenic Cells. Appl. Spectrosc. 2002, 56, 813–819. [Google Scholar] [CrossRef]
- Boere, I.; Schut, T.B.; Boogert, J.V.D.; de Bruin, R.; Puppels, G. Use of fibre optic probes for detection of Barrett’s epithelium in the rat oesophagus by Raman spectroscopy. Vib. Spectrosc. 2003, 32, 47–55. [Google Scholar] [CrossRef]
- Kendall, C.; Stone, N.; Shepherd, N.; Geboes, K.; Warren, B.; Bennett, R.; Barr, H. Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett’s oesophagus. J. Pathol. 2003, 200, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Emery, R.; Pitsillides, A.; Clarkin, C.E.; Mahajan, S. Detection of early osteogenic commitment in primary cells using Raman spectroscopy. Analyst 2017, 142, 1962–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, L.L.; Burke, G.A.; McCafferty, M.M.; O’Hare, P.; Modreanu, M.; Boyd, A.R.; Meenan, B.J. Raman spectroscopic monitoring of the osteogenic differentiation of human mesenchymal stem cells. Analyst 2011, 136, 2471–2481. [Google Scholar] [CrossRef]
- Mitchell, A.; Ashton, L.; Yang, X.B.; Goodacre, R.; Smith, A.; Kirkham, J. Detection of early stage changes associated with adipogenesis using R aman spectroscopy under aseptic conditions. Cytom. Part A 2015, 87, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Akram, Z.; Abduljabbar, T.; Abu Hassan, M.I.; Javed, F.; Vohra, F. Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 4801418. [Google Scholar] [CrossRef] [Green Version]
- Dabitao, D.; Margolick, J.B.; Lopez, J.; Bream, J.H. Multiplex measurement of proinflammatory cytokines in human serum: Comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J. Immunol. Methods 2011, 372, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Miranda, T.S.; Figueiredo, N.D.F.; Figueiredo, L.C.; da Silva, H.D.P.; Rocha, F.R.G.; Duarte, P.M. Cytokine profiles of healthy and diseased sites in individuals with periodontitis. Arch. Oral Biol. 2020, 120, 104957. [Google Scholar] [CrossRef]
- Okada, H.; Murakami, S. Cytokine Expression in Periodontal Health and Disease. Crit. Rev. Oral Biol. Med. 1998, 9, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.C.; Raggatt, L.J.; Alexander, K.A.; Pettit, A.R. Unraveling macrophage contributions to bone repair. BoneKEy Rep. 2013, 2, 373. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, F.; Rellick, S.L.; Piedimonte, G.; Akers, S.M.; O’Leary, H.A.; Martin, K.; Craig, M.D.; Gibson, L.F. Neurotrophins Regulate Bone Marrow Stromal Cell IL-6 Expression through the MAPK Pathway. PLoS ONE 2010, 5, e9690. [Google Scholar] [CrossRef] [Green Version]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016, 1318256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, T.-J.; Gerstenfeld, L.C.; Einhorn, T.A. Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily During Murine Fracture Healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kolar, P.; Gaber, T.; Perka, C.; Duda, G.N.; Buttgereit, F. Human Early Fracture Hematoma Is Characterized by Inflammation and Hypoxia. Clin. Orthop. Relat. Res. 2011, 469, 3118–3126. [Google Scholar] [CrossRef] [Green Version]
- E DeForge, L.; Kenney, J.S.; Jones, M.L.; Warren, J.S.; Remick, D.G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 1992, 148, 2133–2141. [Google Scholar]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; Macdonald, K.L.; Speert, D.P.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.C.J.V.D.; Jansen, B.J.H.; Siebers-Vermeulen, K.G.C.; Roelofs, H.; Figdor, C.G.; Adema, G.J.; Torensma, R. Mesenchymal stem cells respond to TNF but do not produce TNF. J. Leukoc. Biol. 2009, 87, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, M.K.; Jung, M.; Kim, S.H.; Lee, S.R.; Park, K.H.; Kim, N.H.; Kim, H.H.; Park, Y.G. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp. Ther. Med. 2013, 6, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.K.; Park, H.R.; Lee, J.S.; Chung, T.S.; Chung, H.Y.; Chung, J. Down-regulation of iNOS and TNF-α expression by kaempferol via NF-κB inactivation in aged rat gingival tissues. Biogerontology 2007, 8, 399–408. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banavar, S.R.; Rawal, S.Y.; Pulikkotil, S.J.; Daood, U.; Paterson, I.C.; Davamani, F.A.; Kajiya, M.; Kurihara, H.; Khoo, S.P.; Tan, E.L. 3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential. J. Pers. Med. 2021, 11, 528. https://doi.org/10.3390/jpm11060528
Banavar SR, Rawal SY, Pulikkotil SJ, Daood U, Paterson IC, Davamani FA, Kajiya M, Kurihara H, Khoo SP, Tan EL. 3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential. Journal of Personalized Medicine. 2021; 11(6):528. https://doi.org/10.3390/jpm11060528
Chicago/Turabian StyleBanavar, Spoorthi Ravi, Swati Yeshwant Rawal, Shaju Jacob Pulikkotil, Umer Daood, Ian C. Paterson, Fabian Amalraj Davamani, Mikihito Kajiya, Hidemi Kurihara, Suan Phaik Khoo, and Eng Lai Tan. 2021. "3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential" Journal of Personalized Medicine 11, no. 6: 528. https://doi.org/10.3390/jpm11060528
APA StyleBanavar, S. R., Rawal, S. Y., Pulikkotil, S. J., Daood, U., Paterson, I. C., Davamani, F. A., Kajiya, M., Kurihara, H., Khoo, S. P., & Tan, E. L. (2021). 3D Clumps/Extracellular Matrix Complexes of Periodontal Ligament Stem Cells Ameliorate the Attenuating Effects of LPS on Proliferation and Osteogenic Potential. Journal of Personalized Medicine, 11(6), 528. https://doi.org/10.3390/jpm11060528