(Z)-Endoxifen and Early Recurrence of Breast Cancer: An Explorative Analysis in a Prospective Brazilian Study
Abstract
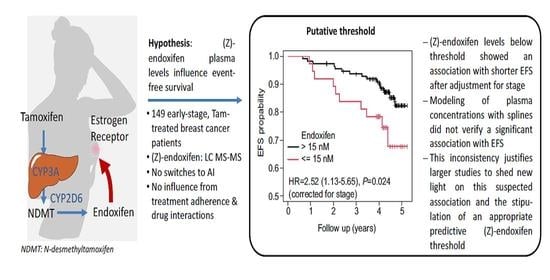
:1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Tamoxifen Treatment
2.2. Assessment of Treatment Adherence and Drug Interactions
2.3. CYP2D6 Polymorphism Genotyping and Quantification of Plasma (Z)-Endoxifen Levels
2.4. Survival Analysis
3. Results
3.1. Patient Characteristics
3.2. Associations of (Z)-Endoxifen Levels with Clinical, Pathological, and CYP2D6 Phenotype Characteristics
3.3. Associations of Clinical and Pathological Variables and CYP2D6 Metabolism Phenotypes with Clinical Outcomes
3.4. Associations of (Z)-Endoxifen Levels with Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Cecchini, R.S.; Cronin, W.M.; Robidoux, A.; Bevers, T.B.; Kavanah, M.T.; Atkins, J.N.; Margolese, R.G.; et al. Tamoxifen for the Prevention of Breast Cancer: Current Status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. JNCI J. Natl. Cancer Inst. 2005, 97, 1652–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.A.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, B. Estrogen Receptor Pathway: Resistance to Endocrine Therapy and New Therapeutic Approaches. Clin. Cancer Res. 2006, 12, 4790–4793. [Google Scholar] [CrossRef] [Green Version]
- Goetz, M.P.; Knox, S.K.; Suman, V.J.; Rae, J.M.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C.; Couch, F.J.; Lingle, W.L.; et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res. Treat. 2007, 101, 113–121. [Google Scholar] [CrossRef]
- McCowan, C.; Shearer, J.; Donnan, P.T.; Dewar, J.A.; Crilly, M.; Thompson, A.M.; Fahey, T. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br. J. Cancer 2008, 99, 1763–1768. [Google Scholar] [CrossRef] [Green Version]
- Chirgwin, J.H.; Giobbie-Hurder, A.; Coates, A.S.; Price, K.N.; Ejlertsen, B.; Debled, M.; Gelber, R.D.; Goldhirsch, A.; Smith, I.; Rabaglio, M.; et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J. Clin. Oncol. 2016, 34, 2452–2459. [Google Scholar] [CrossRef]
- Stearns, V.; Johnson, M.D.; Rae, J.M.; Morocho, A.; Novielli, A.; Bhargava, P.; Hayes, D.F.; Desta, Z.; Flockhart, D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 2003, 95, 1758–1764. [Google Scholar] [CrossRef]
- Saladores, P.; Mürdter, T.; Eccles, D.; Chowbay, B.; Zgheib, N.K.; Winter, S.; Ganchev, B.; Eccles, B.; Gerty, S.; Tfayli, A.; et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharm. J. 2015, 15, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Helland, T.; Henne, N.; Bifulco, E.; Naume, B.; Borgen, E.; Kristensen, V.N.; Kvaløy, J.T.; Lash, T.L.; Alnæs, G.I.G.; van Schaik, R.H.; et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017, 19, 125. [Google Scholar] [CrossRef]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen Metabolite Concentrations, CYP2D6 Genotype, and Breast Cancer Outcomes. Clin. Pharmacol. Ther. 2011, 89, 718–725. [Google Scholar] [CrossRef]
- Desta, Z.; Ward, B.A.; Soukhova, N.V.; Flockhart, D.A. Comprehensive Evaluation of Tamoxifen Sequential Biotransformation by the Human Cytochrome P450 System in Vitro: Prominent Roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 2004, 310, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Schroth, W.; Winter, S.; Mürdter, T.; Schaeffeler, E.; Eccles, D.; Eccles, B.; Chowbay, B.; Khor, C.C.; Tfayli, A.; Zgheib, N.K.; et al. Improved Prediction of Endoxifen Metabolism by CYP2D6 Genotype in Breast Cancer Patients Treated with Tamoxifen. Front. Pharmacol. 2017, 8, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mürdter, T.E.; Schroth, W.; Bacchus-Gerybadze, L.; Winter, S.; Heinkele, G.; Simon, W.; Fasching, P.A.; Fehm, T.; The German Tamoxifen and AI Clinicians Group; Eichelbaum, M.; et al. Activity Levels of Tamoxifen Metabolites at the Estrogen Receptor and the Impact of Genetic Polymorphisms of Phase I and II Enzymes on Their Concentration Levels in Plasma. Clin. Pharmacol. Ther. 2011, 89, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Helland, T.; Alsomairy, S.; Lin, C.; Søiland, H.; Mellgren, G.; Hertz, D.L. Generating a Precision Endoxifen Prediction Algorithm to Advance Personalized Tamoxifen Treatment in Patients with Breast Cancer. J. Pers. Med. 2021, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Ingle, J.N. CYP2D6 Genotype and Tamoxifen: Considerations for Proper Nonprospective Studies. Clin. Pharmacol. Ther. 2014, 96, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauch, H.; Schwab, M. Prediction of tamoxifen outcome by genetic variation of CYP2D6 in post-menopausal women with early breast cancer: Tamoxifen CYP2D6 pharmacogenetics and early breast cancer. Br. J. Clin. Pharmacol. 2014, 77, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Hertz, D.L.; Rae, J.M. One step at a time: CYP2D6 guided tamoxifen treatment awaits convincing evidence of clinical validity. Pharmacogenomics 2016, 17, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Mulder, T.A.M.; de With, M.; del Re, M.; Danesi, R.; Mathijssen, R.H.J.; van Schaik, R.H.N. Clinical CYP2D6 Genotyping to Personalize Adjuvant Tamoxifen Treatment in ER-Positive Breast Cancer Patients: Current Status of a Controversy. Cancers 2021, 13, 771. [Google Scholar] [CrossRef]
- Nardin, J.M.; Schroth, W.; Almeida, T.A.; Mürdter, T.; Picolotto, S.; Vendramini, E.C.L.; Hoppe, R.; Kogin, J.P.; Miqueleto, D.; De Moraes, S.D.R.; et al. The Influences of Adherence to Tamoxifen and CYP2D6 Pharmacogenetics on Plasma Concentrations of the Active Metabolite (Z)-Endoxifen in Breast Cancer. Clin. Transl. Sci. 2019, 13, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Edge, S.B.; Compton, C.C.; American Joint Committee on Cancer (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; 648p. [Google Scholar]
- Eble, J.N.; Sauter, G.; Epstein, J.; Sesterhenn, I. (Eds.) Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; (World Health Organization Classification of Tumours); IARC Press: Lyon, France, 2006; 359p. [Google Scholar]
- Morisky, D.E.; Green, L.W.; Levine, D.M. Concurrent and Predictive Validity of a Self-reported Measure of Medication Adherence. Med. Care 1986, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Munhoz, E.C.; Nardin, J.M.; Carneiro, M.B. Assessment of imatinib mesylate adherence of patients with chronic myeloid leukemia. Rev. Bras. Farm. Hosp. Serv. Saúde 2013, 4, 6–12. [Google Scholar]
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.-J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; McLeod, H.L.; Ratain, M.J.; Zembutsu, H.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin. Pharmacol. Ther. 2018, 103, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Harrell, J. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 23 February 2022).
- Love, R.R.; Desta, Z.; Flockhart, D.; Skaar, T.; Ogburn, E.T.; Ramamoorthy, A.; Uy, G.B.; Laudico, A.V.; Van Dinh, N.; Quang, L.H.; et al. CYP2D6 genotypes, endoxifen levels, and disease recurrence in 224 Filipino and Vietnamese women receiving adjuvant tamoxifen for operable breast cancer. SpringerPlus 2013, 2, 52. [Google Scholar] [CrossRef] [Green Version]
- Braal, C.L.; Beijnen, J.H.; Koolen, S.L.W.; Oomen-de Hoop, E.; Steeghs, N.; Jager, A.; Huitema, A.D.R.; Mathijssen, R.H.J. Relevance of Endoxifen Concentrations: Absence of Evidence Is Not Evidence of Absence. J. Clin. Oncol. 2019, 37, 1980–1981. [Google Scholar] [CrossRef]


| Characteristic | Category | Total (%) | (Z)-Endoxifen (nM) | pa | |
|---|---|---|---|---|---|
| Median | Range | ||||
| Age (years) | >69 | 28 (18.9) | 27.8 | 4.0–61.6 | 0.19 |
| >49–69 | 59 (39.9) | 24.3 | 4.0–54.9 | ||
| <49 | 61 (41.2) | 24.4 | 4.4–48.8 | ||
| Ethnicity | White | 111 (74.5) | 24.9 | 4.0–67.0 | 0.08 |
| Black | 9 (6.0) | 18.8 | 10.1–40.8 | ||
| Asian/Indian | 4 (2.7) | 38.7 | 41.5–61.6 | ||
| Mixed-race | 25 (16.8) | 24.4 | 4.4–52.2 | ||
| BMI (kg/m2) | ≤30 | 61 (70.1) | 24.4 | 4.4–61.6 | 0.38 |
| >30 | 26 (29.9) | 20.1 | 7.2–53.0 | ||
| Histology | Ductal carcinoma | 122 (81.9) | 24.7 | 5.7–54.9 | 0.98 |
| Lobular carcinoma | 12 (8.1) | 23.8 | 4.0–66.7 | ||
| Others | 15 (10.1) | 24.9 | 4.4–61.6 | ||
| Staging | I | 50 (33.6) | 24.9 | 6.1–61.6 | 0.84 |
| II | 74 (49.7) | 24.4 | 4.0–52.6 | ||
| III | 24 (16.1) | 28.6 | 5.7–67.0 | ||
| Molecular subtype | Luminal A | 48 (32.4) | 26.8 | 4.0–67.0 | 0.23 |
| Luminal B | 82 (55.4) | 24.3 | 6.3–61.6 | ||
| HER2-positive | 18 (12.2) | 24.3 | 5.7–45.1 | ||
| Ki67 (%) | ≤14 | 49 (33.1) | 27.1 | 4.0–67.0 | 0.065 |
| >14 | 99 (66.9) | 24.2 | 5.7–61.6 | ||
| Chemotherapy | Yes | 94 (63.1) | 24.7 | 4.4–67.0 | 0.75 |
| No | 55 (36.9) | 24.9 | 4.0–61.6 | ||
| Pathologic complete response | Yes | 9 (16.4) | 24.3 | 10.8–34.9 | 0.78 |
| No | 46 (83.6) | 25.1 | 4.4–67.0 | ||
| CYP2D6 phenotype class | PM | 4 (2.7) | 7.8 | 4.0–67.0 | 9.8 × 10−6 |
| IM | 48 (32.2) | 16.3 | 5.7–51.3 | ||
| NM | 91 (61.1) | 27.6 | 4.4–54.9 | ||
| UM | 6 (4.0) | 38.0 | 28.4–61.6 | ||
| CYP2D6 class combined | PM/IM | 52 (34.9) | 15.6 | 4.0–67.0 | 2.6 × 10−6 |
| NM/UM | 97 (65.1) | 28.2 | 4.4–61.6 | ||
| Factor | Level | Total (n) | Events [n (%)] | HR | 95% CI | p |
|---|---|---|---|---|---|---|
| Tumor stage | I | 50 | 3 (6) | Stage I vs. II: 0.33 Stage III vs. II: 3.71 | 0.09–1.17 1.59–8.72 | 0.0003 |
| II | 74 | 12 (16.2) | ||||
| III | 23 | 10 (43.4) | ||||
| (Z)-endoxifen (nM) 1 | ≤15 | 36 | 10 (27.8) | ≤15 vs. >15 2.52 | 1.13–5.65 | 0.024 |
| >15 | 111 | 15 (13.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, T.; Schroth, W.; Nardin, J.; Mürdter, T.E.; Winter, S.; Picolotto, S.; Hoppe, R.; Kogin, J.; Gaio, E.; Dasenbrock, A.; et al. (Z)-Endoxifen and Early Recurrence of Breast Cancer: An Explorative Analysis in a Prospective Brazilian Study. J. Pers. Med. 2022, 12, 511. https://doi.org/10.3390/jpm12040511
Almeida T, Schroth W, Nardin J, Mürdter TE, Winter S, Picolotto S, Hoppe R, Kogin J, Gaio E, Dasenbrock A, et al. (Z)-Endoxifen and Early Recurrence of Breast Cancer: An Explorative Analysis in a Prospective Brazilian Study. Journal of Personalized Medicine. 2022; 12(4):511. https://doi.org/10.3390/jpm12040511
Chicago/Turabian StyleAlmeida, Thais, Werner Schroth, Jeanine Nardin, Thomas E. Mürdter, Stefan Winter, Solane Picolotto, Reiner Hoppe, Jenifer Kogin, Elisa Gaio, Angela Dasenbrock, and et al. 2022. "(Z)-Endoxifen and Early Recurrence of Breast Cancer: An Explorative Analysis in a Prospective Brazilian Study" Journal of Personalized Medicine 12, no. 4: 511. https://doi.org/10.3390/jpm12040511
APA StyleAlmeida, T., Schroth, W., Nardin, J., Mürdter, T. E., Winter, S., Picolotto, S., Hoppe, R., Kogin, J., Gaio, E., Dasenbrock, A., Skrsypcsak, R. C., de Noronha, L., Schwab, M., Brauch, H., & Casali-da-Rocha, J. C. (2022). (Z)-Endoxifen and Early Recurrence of Breast Cancer: An Explorative Analysis in a Prospective Brazilian Study. Journal of Personalized Medicine, 12(4), 511. https://doi.org/10.3390/jpm12040511