Analysis of Effect of Intensity of Aerobic Exercise on Cognitive and Motor Functions and Neurotrophic Factor Expression Patterns in an Alzheimer’s Disease Rat Model
Abstract
:1. Introduction
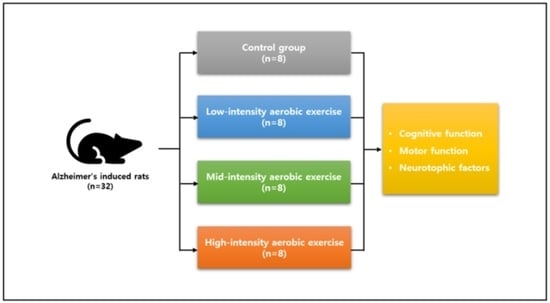
2. Methods
2.1. Experimental Animals
2.2. Induction of Alzheimer’s Disease
2.3. Treadmill Exercise Protocol
2.4. Cognitive Function Test
Eight-Arm Radial Maze Test
2.5. Motor Function Test
2.5.1. Beam-Walking Test
2.5.2. Ladder Rung Walking Test
2.6. Histological Examination
2.6.1. Brain Extraction and Tissue Fixation
2.6.2. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Comparison of Cognitive Function Results between and within Groups
3.2. Comparison of Motor Function Results between and within Groups
3.3. Comparison of Neurotrophic Factor Expression Results between Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagattini, C.; Zanni, M.; Barocco, F.; Caffarra, P.; Brignani, D.; Miniussi, C.; Defanti, C.A. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020, 13, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Quan, M.; Fu, Y.; Zhao, T.; Li, Y.; Wei, C.; Tang, Y.; Qin, Q.; Wang, F.; Qiao, Y.; et al. Dementia in China: Epidemiology, clinical management, and research advances. Lancet Neurol. 2020, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sopina, E.; Sørensen, J.; Beyer, N.; Hasselbalch, S.G.; Waldemar, G. Cost-effectiveness of a randomised trial of physical activity in Alzheimer’s disease: A secondary analysis exploring patient and proxy-reported health-related quality of life measures in Denmark. BMJ Open 2017, 7, e015217. [Google Scholar] [CrossRef] [PubMed]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s disease diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Will, B.; Galani, R.; Kelche, C.; Rosenzweig, M.R. Recovery from brain injury in animals: Relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002). Prog. Neurobiol. 2004, 72, 167–182. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch. Neurol. 2006, 63, 1763–1769. [Google Scholar] [CrossRef]
- Gould, E.; Gross, C.G. Neurogenesis in adult mammals: Some progress and problems. J. Neurosci. 2002, 22, 619–623. [Google Scholar] [CrossRef]
- Mattson, M.P.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef]
- Pareja-Galeano, H.; Brioche, T.; Sanchis-Gomar, F.; Montal, A.; Jovaní, C.; Martínez-Costa, C.; Gomez-Cabrera, M.C.; Viña, J. Impact of exercise training on neuroplasticity-related growth factors in adolescents. J. Musculoskelet. Neuronal Interact. 2013, 13, 368–371. [Google Scholar]
- Venediktov, A.A.; Bushueva, O.Y.; Kudryavtseva, V.A.; Kuzmin, E.A.; Moiseeva, A.V.; Baldycheva, A.; Meglinski, I.; Piavchenko, G.A. Closest horizons of Hsp70 engagement to manage neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1230436. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Shahid, M.; Ansari, J.; Ahanger, I.A.; Hassan, I.; Islam, A. Recent Advancement in Therapeutic Strategies for Alzheimer’s Disease: Insights from Clinical Trials. Ageing Res. Rev. 2023, 92, 102113. [Google Scholar] [CrossRef]
- Oliver, C.; Li, H.; Biswas, B.; Woodstoke, D.; Blackman, J.; Butters, A.; Drew, C.; Gabb, V.; Harding, S.; Hoyos, C.M.; et al. A systematic review on adherence to continuous positive airway pressure (CPAP) treatment for obstructive sleep apnoea (OSA) in individuals with mild cognitive impairment and Alzheimer’s disease dementia. Sleep Med. Rev. 2023, 73, 101869. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Quindós-Rubial, M.; Fiuza-Luces, C.; Cristi-Montero, C.; Emanuele, E.; Garatachea, N.; Lucia, A. Physical activity and Alzheimer disease: A protective association. Mayo Clin. Proc. 2016, 91, 999–1020. [Google Scholar] [CrossRef]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, 2015, Cd006489. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Baktir, M.A.; Das, D.; Lin, B.; Salehi, A. The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys. Ther. 2015, 95, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Choi, D.H.; Kwon, K.C.; Hwang, D.J.; Koo, J.H.; Um, H.S.; Song, H.S.; Kim, J.S.; Jang, Y.; Cho, J.Y. Treadmill exercise alleviates brain iron dyshomeostasis accelerating neuronal amyloid-β production, neuronal cell death, and cognitive impairment in transgenic mice model of Alzheimer’s disease. Mol. Neurobiol. 2021, 58, 3208–3223. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Q.W.; Xia, J.; Zhang, X.L.; Li, B.X.; Yin, L.Y.; Xu, B. Treadmill exercise attenuates Aβ-induced mitochondrial dysfunction and enhances mitophagy activity in APP/PS1 transgenic mice. Neurochem. Res. 2020, 45, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Sobol, N.A.; Dall, C.H.; Høgh, P.; Hoffmann, K.; Frederiksen, K.S.; Vogel, A.; Siersma, V.; Waldemar, G.; Hasselbalch, S.G.; Beyer, N. Change in fitness and the relation to change in cognition and neuropsychiatric symptoms after aerobic exercise in patients with mild Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Dugravot, A.; Dartigues, J.F.; Abell, J.; Elbaz, A.; Kivimäki, M.; Singh-Manoux, A. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 2017, 357, j2709. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, E.D.; Morris, J.K.; Watts, A.; Perry, M.; Clutton, J.; Van Sciver, A.; Kamat, A.S.; Mahnken, J.; Hunt, S.L.; Townley, R.; et al. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: A 1-year randomized controlled trial. PLoS ONE 2021, 16, e0244893. [Google Scholar] [CrossRef]
- Garnier, C.; Falempin, M.; Canu, M.H. A 3D analysis of fore- and hindlimb motion during locomotion: Comparison of overground and ladder walking in rats. Behav. Brain Res. 2008, 186, 57–65. [Google Scholar] [CrossRef]
- Su, Y.; Wang, D.; Liu, N.; Yang, J.; Sun, R.; Zhang, Z. Clostridium butyricum improves cognitive dysfunction in ICV-STZ-induced Alzheimer’s disease mice via suppressing TLR4 signaling pathway through the gut-brain axis. PLoS ONE 2023, 18, e0286086. [Google Scholar] [CrossRef]
- Jung, S.W.; Han, O.K.; Kim, S.J. Increased expression of β amyloid precursor gene in the hippocampus of streptozotocin-induced diabetic mice with memory deficit and anxiety induction. J. Neural Transm. 2010, 117, 1411–1418. [Google Scholar] [CrossRef]
- Ishrat, T.; Parveen, K.; Khan, M.M.; Khuwaja, G.; Khan, M.B.; Yousuf, S.; Ahmad, A.; Shrivastav, P.; Islam, F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009, 1281, 117–127. [Google Scholar] [CrossRef]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef]
- Shepherd, R.E.; Gollnick, P.D. Oxygen uptake of rats at different work intensities. Pflugers Arch. 1976, 362, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, S.; Hida, H.; Masuda, T.; Misumi, S.; Kim, T.S.; Nishino, H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience 2007, 144, 920–933. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. The ladder rung walking task: A scoring system and its practical application. J. Vis. Exp. 2009, 28, 1204. [Google Scholar] [CrossRef]
- Farzi, M.A.; Sadigh-Eteghad, S.; Ebrahimi, K.; Talebi, M. Exercise improves recognition memory and acetylcholinesterase activity in the beta amyloid-induced rat model of Alzheimer’s disease. Ann. Neurosci. 2019, 25, 121–125. [Google Scholar] [CrossRef]
- Mu, L.; Cai, J.; Gu, B.; Yu, L.; Li, C.; Liu, Q.S.; Zhao, L. Treadmill exercise prevents decline in spatial learning and memory in 3×Tg-AD mice through enhancement of structural synaptic plasticity of the hippocampus and prefrontal cortex. Cells 2022, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Dare, L.R.; Garcia, A.; Soares, C.B.; Lopes, L.; Neves, B.S.; Dias, D.V.; Mello-Carpes, P.B. The reversal of memory deficits in an Alzheimer’s disease model using physical and cognitive exercise. Front. Behav. Neurosci. 2020, 14, 152. [Google Scholar] [CrossRef]
- Yu, F.; Vock, D.M.; Zhang, L.; Salisbury, D.; Nelson, N.W.; Chow, L.S.; Smith, G.; Barclay, T.R.; Dysken, M.; Wyman, J.F. Cognitive effects of aerobic exercise in Alzheimer’s disease: A pilot randomized controlled trial. J. Alzheimer’s Dis. 2021, 80, 233–244. [Google Scholar] [CrossRef]
- Yang, S.Y.; Shan, C.L.; Qing, H.; Wang, W.; Zhu, Y.; Yin, M.M.; Machado, S.; Yuan, T.F.; Wu, T. The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol. Disord. Drug Targets 2015, 14, 1292–1297. [Google Scholar] [CrossRef]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Physical and motor fitness are both related to cognition in old age. Eur. J. Neurosci. 2010, 31, 167–176. [Google Scholar] [CrossRef]
- Koo, J.H.; Kang, E.B.; Oh, Y.S.; Yang, D.S.; Cho, J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 288, 142–152. [Google Scholar] [CrossRef]
- Riek-Burchardt, M.; Henrich-Noack, P.; Metz, G.A.; Reymann, K.G. Detection of chronic sensorimotor impairments in the ladder rung walking task in rats with endothelin-1-induced mild focal ischemia. J. Neurosci. Methods 2004, 137, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ong, R.; Burnette, D. The influence of ethnicity and culture on dementia caregiving: A review of empirical studies on Chinese Americans. Am. J. Alzheimer’s Dis. Other Dement. 2012, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Hortobágyi, T.; Scherder, E.J.; van Heuvelen, M.J. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: A randomized, controlled trial. Am. J. Geriatr. Psychiatry 2015, 23, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Colcombe, S. Fitness effects on the cognitive function of older adults: A meta-analytic study-revisited. Perspect. Psychol. Sci. 2018, 13, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xie, Y.; Tessarollo, L.; Xu, B. Midbrain-derived neurotrophins support survival of immature striatal projection neurons. J. Neurosci. 2013, 33, 3363–3369. [Google Scholar] [CrossRef]
- Rauskolb, S.; Zagrebelsky, M.; Dreznjak, A.; Deogracias, R.; Matsumoto, T.; Wiese, S.; Erne, B.; Sendtner, M.; Schaeren-Wiemers, N.; Korte, M.; et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J. Neurosci. 2010, 30, 1739–1749. [Google Scholar] [CrossRef]
- Lübke, J.H.; Idoon, F.; Mohasel-Roodi, M.; Alipour, F.; Hami, J.; Ehteshampour, A.; Mostafaee, H.; Sadeghi, A. Neurotrophic factors in Alzheimer’s disease: Pathogenesis and therapy. Acta Neurobiol. Exp. 2021, 81, 314–327. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Madathil, S.K.; Carlson, S.W.; Brelsfoard, J.M.; Ye, P.; D’Ercole, A.J.; Saatman, K.E. Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS ONE 2013, 8, e67204. [Google Scholar] [CrossRef]
- Kang, K.; Bai, J.; Zhong, S.; Zhang, R.; Zhang, X.; Xu, Y.; Zhao, M.; Zhao, C.; Zhou, Z. Down-regulation of insulin like growth factor 1 involved in Alzheimer’s disease via MAPK, Ras, and FoxO signaling pathways. Oxid. Med. Cell Longev. 2022, 2022, 8169981. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Civelek, S.; Konukoglu, D.; Erdenen, F.; Uzun, H. Serum neurotrophic factor levels in patients with type 2 diabetes mellitus: Relationship to metabolic syndrome components. Clin. Lab. 2013, 59, 369–374. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 2016, 131, 142–154. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 569–579. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Jin, L.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Radak, Z.; Toldy, A.; Szabo, Z.; Siamilis, S.; Nyakas, C.; Silye, G.; Jakus, J.; Goto, S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem. Int. 2006, 49, 387–392. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Luan, X.; Ding, Y.H.; Lai, Q.; Rafols, J.A.; Phillis, J.W.; Clark, J.C.; Diaz, F.G. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience 2004, 124, 583–591. [Google Scholar] [CrossRef]
- Hopkins, M.E.; Bucci, D.J. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiol. Learn. Mem. 2010, 94, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Klintsova, A.Y.; Dickson, E.; Yoshida, R.; Greenough, W.T. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004, 1028, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Rojas Vega, S.; Hollmann, W.; Vera Wahrmann, B.; Strüder, H.K. pH buffering does not influence BDNF responses to exercise. Int. J. Sports Med. 2012, 33, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]




| Variable | Control Group | Group I | Group II | Group III | F (p) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| Radial 8-arm maze time index (second) | 280.25 (16.49) | 286.12 (15.18) | 277.63 (13.96) | 249.13 (14.04) | 282.13 (11.25) | 255.32 (10.45) | 279.38 (10.61) | 234.13 ‡ (12.18) | 33.631 (0.025 *) |
| Radial 8-arm maze error index (number) | 5.63 (1.69) | 5.38 (1.52) | 6.00 (1.69) | 2.50 ‡ (1.31) | 6.38 (1.41) | 3.63 ‡ (1.18) | 5.85 (1.04) | 2.25 ‡ (1.26) | 8.893 (0.000 *) |
| Radial 8-arm maze find index (number) | 3.48 (0.52) | 3.50 (0.73) | 3.75 (0.46) | 6.38 ‡ (0.52) | 3.25 (0.84) | 6.00 ‡ (0.76) | 3.38 (0.63) | 5.95 ‡ (0.46) | 9.241 (0.000 *) |
| Variable | Control Group | Group I | Group II | Group III | F (p) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| Beam-walking test (score) | 2.79 (0.42) | 3.08 (0.61) | 2.67 (0.87) | 4.52 ‡ (0.24) | 2.54 (0.78) | 4.24 ‡ (0.38) | 2.33 (0.36) | 4.25 ‡ (0.39) | 6.367 (0.002 *) |
| Ladder rung walking test (score) | 28.65 (4.48) | 28.13 (5.34) | 26.40 (5.87) | 10.42 ‡ (4.26) | 25.44 (4.16) | 9.86 ‡ (3.92) | 26.87 (5.65) | 12.50 ‡ (3.15) | 9.890 (0.000 *) |
| Variable | Control Group | Group I | Group II | Group III | F (p) |
|---|---|---|---|---|---|
| IGF-1 (pixels) | 566.00 (220.26) | 18,782.75 (835.37) | 13,213.25 (691.47) | 14,016.63 (719.59) | 1261.93 (0.000 *) |
| NGF (pixels) | 1728.25 (198.86) | 31,503.50 (1253.11) | 35,315.63 (1456.08) | 28,126.88 (955.53) | 1015.31 (0.000 *) |
| BDNF (pixels) | 3672.25 (400.90) | 9311.50 (939.24) | 8733.50 (736.01) | 23,787.51 (916.93) | 987.89 (0.000 *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y.; Im, S.-C.; Kang, N.-Y.; Kim, K. Analysis of Effect of Intensity of Aerobic Exercise on Cognitive and Motor Functions and Neurotrophic Factor Expression Patterns in an Alzheimer’s Disease Rat Model. J. Pers. Med. 2023, 13, 1622. https://doi.org/10.3390/jpm13111622
Lee D-Y, Im S-C, Kang N-Y, Kim K. Analysis of Effect of Intensity of Aerobic Exercise on Cognitive and Motor Functions and Neurotrophic Factor Expression Patterns in an Alzheimer’s Disease Rat Model. Journal of Personalized Medicine. 2023; 13(11):1622. https://doi.org/10.3390/jpm13111622
Chicago/Turabian StyleLee, Do-Youn, Sang-Cheol Im, Na-Yeon Kang, and Kyoung Kim. 2023. "Analysis of Effect of Intensity of Aerobic Exercise on Cognitive and Motor Functions and Neurotrophic Factor Expression Patterns in an Alzheimer’s Disease Rat Model" Journal of Personalized Medicine 13, no. 11: 1622. https://doi.org/10.3390/jpm13111622
APA StyleLee, D. -Y., Im, S. -C., Kang, N. -Y., & Kim, K. (2023). Analysis of Effect of Intensity of Aerobic Exercise on Cognitive and Motor Functions and Neurotrophic Factor Expression Patterns in an Alzheimer’s Disease Rat Model. Journal of Personalized Medicine, 13(11), 1622. https://doi.org/10.3390/jpm13111622