The Impact of Water on the Tribological Behavior of Lubricating Grease Based on Calcium Carbonate Polymorphs
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Preparation
2.2. Sample Characterization
2.3. Tribological Performance
3. Results and Discussion
3.1. Physiochemical Properties of CSCG
3.2. Rheological Analysis
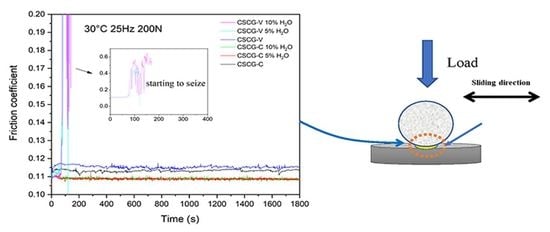
3.3. Tribological Evaluation
3.4. Surface Analysis
3.5. Possible Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mortier, R.M.; Fox, M.F.; Orszulik, S.T. Chemistry and Technology of Lubricants; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lugt, P.M. A review on grease lubrication in rolling bearings. Tribol. Trans. 2009, 52, 470–480. [Google Scholar] [CrossRef]
- Lugt, P.M. Modern advancements in lubricating grease technology. Tribol. Int. 2016, 97, 467–477. [Google Scholar] [CrossRef]
- Varebberg, M.; Kligerman, Y.; Halperin, G.; Nakad, S.; Kasem, K. Assessing workability of greased bearings after long-term storage. Friction 2019, 7, 489–496. [Google Scholar] [CrossRef]
- Cen, H.; Lugt, P.M. Film thickness in a grease lubricated ball bearing. Tribol. Int. 2019, 134, 26–35. [Google Scholar] [CrossRef]
- Wang, D.F.; Yang, J.L.; Wei, P.C.; Pu, W. A mixed EHL model of grease lubrication considering surface roughness and the study of friction behavior. Tribol. Int. 2021, 154, 106710. [Google Scholar] [CrossRef]
- Grebe, M.; Ruland, M. Influence of mechanical, thermal, oxidative and catalytic processes on thickener structure and thus on the service life of rolling bearings. Lubricants 2022, 10, 77. [Google Scholar] [CrossRef]
- Akchurin, A.; Ende, D.V.N.; Lugt, P.M. Modeling impact of grease mechanical ageing on bleed and permeability in rolling bearings. Tribol. Int. 2022, 170, 107507. [Google Scholar] [CrossRef]
- Gurt, A.; Khonsari, M. An overview of grease water resistance. Lubricants 2020, 8, 86. [Google Scholar] [CrossRef]
- Cyriac, F.; Lugt, P.M.; Bosman, R. The impact of water on the yield stress and startup torque of lubricating greases. Tribol. Trans. 2017, 60, 824–831. [Google Scholar] [CrossRef]
- Cyriac, F.; Lugt, P.M.; Bosman, R. Impact of water on the rheology of lubricating greases. Tribol. Trans. 2015, 59, 679–689. [Google Scholar] [CrossRef]
- Mistry, A. Performance of lubricating greases in the presence of water. NLGI Spokesman. 2005, 68, 8–15. [Google Scholar]
- Cyriac, F.; Lugt, P.M.; Bosman, R.; Venner, C.H. Impact of water on EHL film thickness of lubricating greases in rolling point contact. Tribol. Lett. 2016, 61, 23. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Bosman, R.; Lugt, P.M. On the shear stability of dry and water-contaminated calcium sulfonate complex lubricating greases. Tribol. Trans. 2019, 62, 626–634. [Google Scholar] [CrossRef]
- Hudedagaddi, C.B.; Raghav, A.G.; Tortora, A.M.; Veeregowda, D.H. Water molecules influence the lubricity of greases and fuel. Wear 2017, 376, 831–835. [Google Scholar] [CrossRef]
- Denis, R.; Sivik, M. Calcium Sulphonate Grease-Making Processes. NLGI Spokesm. 2009, 73, 30–37. [Google Scholar]
- Macwood, W.; Muir, R. Calcium Sulphonate Grease. One Decade Later. NLGI Spokesm. 1999, 63, 24–27. [Google Scholar]
- Wang, Z.Y.; Xia, Y.Q.; Liu, Z.L. The rheological and tribological properties of calcium sulfonate complex greases. Friction 2015, 3, 28–35. [Google Scholar] [CrossRef]
- Muir, R.J. High Performance Calcium Sulphonate Complex Lubricating Grease. NLGI Spokesm. 1988, 52, 140–146. [Google Scholar]
- Rizvi, S.Q.A. A Comprehensive Review of Lubricant Chemistry, Technology, Selection, and Design; ASTM International Publisher: New York, NY, USA, 2009. [Google Scholar]
- Liu, D.B.; Zhao, G.Q.; Wang, X.B. Tribological performance of lubricating greases based on calcium carbonate polymorphs under the boundary lubrication condition. Tribol. Lett. 2012, 47, 183–194. [Google Scholar] [CrossRef]
- Liu, D.B.; Zhang, M.; Zhao, G.Q.; Wang, X.B. Tribological behavior of amorphous and crystalline overbased calcium sulphonate as additives in lithium complex grease. Tribol. Lett. 2012, 45, 265–273. [Google Scholar] [CrossRef]
- Glasson, S.; Esplnat, D.; Palermo, T. Study of microstructural transformation of overbased calcium sulphonates during friction. Lubr. Sci. 1993, 5, 91–111. [Google Scholar] [CrossRef]
- Gueta, R.; Natan, A.; Addadi, L.; Weiner, S.; Refson, K.; Kronik, L. Local atomic order and infrared spectra of biogenic calcite. Angew. Chem. Int. Ed. 2007, 46, 291–294. [Google Scholar] [CrossRef]
- Gal, A.; Weiner, S.; Addadi, L. The stabilizing effect of silicate on biogenic and synthetic amorphous calcium carbonate. J. Am. Chem. Soc. 2010, 132, 13208–13211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Xiao, S.; Chen, F.; Chen, D.Z.; Fang, J.L.; Zhao, M. Calcium carbonate phase transformations during the carbonation reaction of calcium heavy alkylbenzene sulphonate overbased nanodetergents preparation. J. Colloid Interface Sci. 2011, 359, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.B. Dopamine-induced mineralization of calcium carbonate vaterite microspheres. Langmuir 2010, 26, 14730–14736. [Google Scholar] [CrossRef]
- Xu, N.; Li, W.M.; Zhang, M.; Wang, X.B. Reinforcing effect of Lewis acid–base interaction on the high-temperature colloidal stability and tribological performance of lubricating grease. J. Ind. Eng. Chem. 2017, 46, 157–164. [Google Scholar] [CrossRef]
- Zhang, E.H.; Li, W.M.; Zhao, G.Q.; Wang, Z.; Wang, X.B. A Study on microstructure, friction and rheology of four lithium greases formulated with four different base oils. Tribol. Lett. 2021, 69, 98. [Google Scholar] [CrossRef]
- Ren, J.; Gong, K.L.; Zhao, G.Q.; Wu, X.H.; Wang, X.B. Investigation of the interaction, rheological and tribological properties of bis(pinacolato)diboron with lithium grease. Tribol. Lett. 2021, 69, 166. [Google Scholar]
- Ji, X.B.; Chen, Y.X.; Zhao, G.Q.; Wang, X.B.; Liu, W.M. Tribological properties of CaCO3 nanoparticles as an additive in lithium grease. Tribol. Lett. 2010, 41, 113–119. [Google Scholar] [CrossRef]
- Wager, C.D.; Ring, W.M.; Davids, L.E. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division Press: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Cai, M.R.; Liang, Y.M.; Yao, M.H.; Xia, Y.Q.; Zhou, F.; Liu, W.M. Imidazolium ionic liquids as antiwear and antioxidant additive in poly(ethylene glycol) for steel/steel contacts. ACS Appl. Mater. Interfaces 2010, 2, 870–876. [Google Scholar] [CrossRef]
- Gong, L.F.; Qian, S.H.; Wang, W.; Ni, Z.F.; Tang, L. Influence of nano-additives (nano-PTFE and nano-CaCO3) on tribological properties of food-grade aluminum-based grease. Tribol. Int. 2021, 160, 107014. [Google Scholar] [CrossRef]
- Costello, M.T. Study of surface films of amorphous and crystalline overbased calcium sulphonate by XPS and AES. Tribol. Trans. 2006, 49, 592–597. [Google Scholar] [CrossRef]
- Hanawa, T.; Ukai, H.; Murakami, K. X-ray photoelectron spectroscopy of calcium-ion-implanted titanium. J. Electron. Spectrosc. Relat. Phenom. 1993, 63, 347–354. [Google Scholar] [CrossRef]
- Cizaire, L.; Martin, J.M.; Gresser, E.; Truong Dinh, N.; Heau, C. Tribochemistry of overbased calcium detergents studied by ToFSIMS. Tribol. Lett. 2004, 17, 715–721. [Google Scholar] [CrossRef]
- Palermo, T.; Giasson, S.; Buffeteau, T.; Desbat, B.; Turlet, J.M. Study of deposit and friction films of overbased calcium sulphonate by PM-IRRAS spectroscopy. Lubr. Sci. 1996, 8, 119–127. [Google Scholar] [CrossRef]
- Chinas-Castillo, F.; Spikes, H.A. Film formation by colloidal overbased detergents in lubricated contacts. Tribol. Trans. 2000, 43, 357–366. [Google Scholar] [CrossRef]
- Bosman, B.; Lugt, P.M. The microstructure of calcium sulfonate complex lubricating grease and its change in the presence of water. Tribol. Trans. 2018, 61, 842–849. [Google Scholar] [CrossRef]









| Grease Property | CSCG-V | CSCG-V +5% Water | CSCG-V +10% Water | CSCG-C | CSCG-C +5% Water | CSCG-C +10% Water | Test Standard |
|---|---|---|---|---|---|---|---|
| Base oil | 150BS | 150BS | 150BS | 150BS | 150BS | 150BS | |
| Cone penetration (0.1 mm) | 256 ± 3 | 267 ± 6 | 261 ± 6 | 261 ± 3 | 254 ± 6 | 253 ± 6 | ASTM D 217 |
| Dropping point (°C) | 320 ± 5 | 320 ± 5 | 320 ± 5 | 320 ± 5 | 320 ± 5 | 320 ± 5 | ASTM D 566 |
| Four-ball EP test * | ASTM D 2596 | ||||||
| Last non-seizure load (N) | 980 | 618 | 548 | 980 | 882 | 980 | |
| Weld point (N) | 3920 | 3087 | 2450 | 3920 | 3087 | 3920 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Ma, R.; Zhao, Q.; Zhao, G.; Wang, X. The Impact of Water on the Tribological Behavior of Lubricating Grease Based on Calcium Carbonate Polymorphs. Lubricants 2022, 10, 188. https://doi.org/10.3390/lubricants10080188
Sun L, Ma R, Zhao Q, Zhao G, Wang X. The Impact of Water on the Tribological Behavior of Lubricating Grease Based on Calcium Carbonate Polymorphs. Lubricants. 2022; 10(8):188. https://doi.org/10.3390/lubricants10080188
Chicago/Turabian StyleSun, Longqi, Rui Ma, Qin Zhao, Gaiqing Zhao, and Xiaobo Wang. 2022. "The Impact of Water on the Tribological Behavior of Lubricating Grease Based on Calcium Carbonate Polymorphs" Lubricants 10, no. 8: 188. https://doi.org/10.3390/lubricants10080188
APA StyleSun, L., Ma, R., Zhao, Q., Zhao, G., & Wang, X. (2022). The Impact of Water on the Tribological Behavior of Lubricating Grease Based on Calcium Carbonate Polymorphs. Lubricants, 10(8), 188. https://doi.org/10.3390/lubricants10080188