Influence of Research Factors and Al2O3 Layer Production Parameters on Tribological and Microstructural Properties
Abstract
:1. Introduction
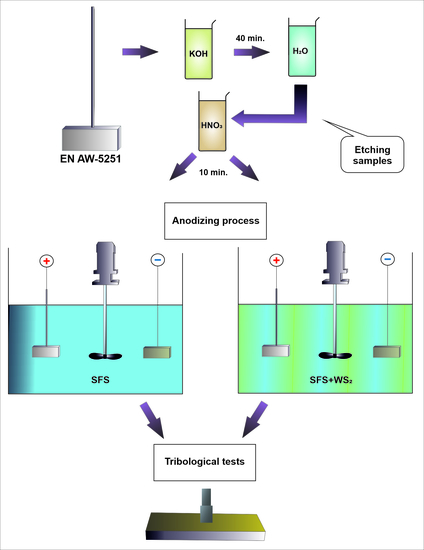
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Analysis of variance has allowed us to establish that the material sputtered (carbon and gold) on the surface before SEM tests has a significant impact on the calculations related to the size of surface nanopores and Al2O3 walls. The carbon sputtering preparation was characterized by about 20% larger nanopore diameters and about 30% larger values of the size of Al2O3 walls in relation to the gold sputtering preparation. The DOE analysis has not shown a significant effect of WS2 doping to the electrolyte on the sizes of Al2O3 walls and nanopores.
- Analysis of variance and Pareto charts shows that both the load and the type of electrolyte have an impact on the values of friction coefficient. Spearman’s rank coefficient correlation shows a strong negative correlation between the coefficient of friction and the load (R = −0.67), and the coefficient of friction and the type of electrolyte (R = −0.51). A very strong positive correlation (R = 0.87) has been shown for the relationship between the mass wear of the TG15 compound and the load. A moderate positive correlation (R = 0.43) has been found between the mass wear parameter of TG15 and the type of electrolyte. The application of the WS2 admixture to the electrolyte slightly reduces the wear of the TG15 polymer pin and increases the coefficient of friction for the tested friction node. This result may be caused by an improved sorption of the material to the oxide layer and increased adhesion.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruera, F.A.; Kramer, G.R.; Vera, L.; Ares, A.E. Low-Cost Nanostructured Coating of Anodic Aluminium Oxide Synthesized in Sulphuric Acid as Electrolyte. Coatings 2021, 11, 309. [Google Scholar] [CrossRef]
- Gombár, M.; Vagaská, A.; Harničárová, M.; Valíček, J.; Kušnerová, M.; Czán, A.; Kmec, J. Experimental analysis of the influence of factors acting on the layer thickness formed by anodic oxidation of aluminium. Coatings 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Buczko, Z.; Olkowicz, K.; Krasucki, J.; Grabowiecki, K.; Osuchowska, E.; Tomassi, P. Superhydrophobic properties of aluminium produced by surface abrasive blasting, anodic oxidation and fatty acid impregnation. Trans. Inst. Met. Finish. 2021, 99, 73–79. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Synthesis and morphological characterization of nanoporous aluminum oxide films by using a single anodization step. Coatings 2019, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.K.; Liao, M.W.; Lee, C.T.; Chang, H.C. Anodization of nanoporous alumina on impurity-induced hemisphere curved surface of aluminum at room temperature. Nanoscale Res. Lett. 2011, 6, 596. [Google Scholar] [CrossRef] [Green Version]
- Skoneczny, W. Effect of the base material condition on the structure and properties of Al2O3 oxide layers. Indian J. Eng. Mater. Sci. 2020, 27, 128–132. [Google Scholar]
- Stȩpniowski, W.J.; Bojar, Z. Synthesis of anodic aluminum oxide (AAO) at relatively high temperatures. Study of the influence of anodization conditions on the alumina structural features. Surf. Coatings Technol. 2011, 206, 265–272. [Google Scholar] [CrossRef]
- Sulka, G.D.; Stepniowski, W.J. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochim. Acta 2009, 54, 3683–3691. [Google Scholar] [CrossRef]
- Kwolek, P.; Drapała, D.; Krupa, K.; Obłój, A.; Tokarski, T.; Sieniawski, J. Mechanical properties of a pulsed anodised 5005 aluminium alloy. Surf. Coatings Technol. 2020, 383, 125233. [Google Scholar] [CrossRef]
- Jensen, F.; Gudla, V.C.; Kongstad, I.; Ambat, R. High frequency pulse anodising of aluminium: Anodising kinetics and optical appearance. Surf. Coatings Technol. 2019, 360, 222–231. [Google Scholar] [CrossRef]
- Bononi, M.; Giovanardi, R.; Bozza, A. Pulsed current hard anodizing of heat treated aluminum alloys: Frequency and current amplitude influence. Surf. Coatings Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Lee, W.; Schwirn, K.; Steinhart, M.; Pippel, E.; Scholz, R.; Gösele, U. Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium. Nat. Nanotechnol. 2008, 3, 234–239. [Google Scholar] [CrossRef]
- Mohammadi, I.; Afshar, A. Modification of nanostructured anodized aluminum coatings by pulse current mode. Surf. Coatings Technol. 2015, 278, 48–55. [Google Scholar] [CrossRef]
- Mohammadi, I.; Ahmadi, S.; Afshar, A. Effect of pulse current parameters on the mechanical and corrosion properties of anodized nanoporous aluminum coatings. Mater. Chem. Phys. 2016, 183, 490–498. [Google Scholar] [CrossRef]
- Chung, C.K.; Zhou, R.X.; Liu, T.Y.; Chang, W.T. Hybrid pulse anodization for the fabrication of porous anodic alumina films from commercial purity (99%) aluminum at room temperature. Nanotechnology 2009, 20, 055301. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Chang, W.T.; Liao, M.W.; Chang, H.C. Effect of pulse voltage and aluminum purity on the characteristics of anodic aluminum oxide using hybrid pulse anodization at room temperature. Thin Solid Films 2011, 519, 4754–4758. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Kessentini, I.; Zouari, S.; Bakir, A.; Bargui, M. Comparative Study of Mechanical and Tribological Properties of Alumina Coatings Formed on 5754 Aluminium Alloy under Various Conditions. Surf. Eng. Appl. Electrochem. 2018, 54, 524–533. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Bara, M.; Barylski, A. Dependence of the Surface Morphology and Micromechanical and Sclerometric Properties of Al2O3 Layers on the Parameters of Anodizing Aluminum Alloy. Materials 2022, 15, 8482. [Google Scholar] [CrossRef]
- Kwolek, P.; Krupa, K.; Obłój, A.; Kocurek, P.; Wierzbińska, M.; Sieniawski, J. Tribological Properties of the Oxide Coatings Produced onto 6061-T6 Aluminum Alloy in the Hard Anodizing Process. J. Mater. Eng. Perform. 2018, 27, 3268–3275. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yi, H.; Wang, H. Analysis and self-lubricating treatment of porous anodic alumina film formed in a compound solution. Appl. Surf. Sci. 2005, 252, 1662–1667. [Google Scholar] [CrossRef]
- Azzi, A.; Maoui, A.; Fatu, A.; Fily, S.; Souchet, D. Experimental study of friction in pneumatic seals. Tribol. Int. 2019, 135, 432–443. [Google Scholar] [CrossRef]
- Chang, H.; Lan, C.W.; Chen, C.H.; Tsung, T.T.; Guo, J. Bin Measurement of frictional force characteristics of pneumatic cylinders under dry and lubricated conditions. Electr. Rev. 2012, 88, 261–264. [Google Scholar]
- Kmita, T.; Bara, M. Surface oxide layers with an increased carbon content for applications in oil-Less tribological systems. Chem. Process Eng. 2012, 33, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Korzekwa, J.; Bara, M.; Kaptacz, S. Al2O3/WS2 Surface Layers Produced on the Basis of Aluminum Alloys for Applications in Oil-Free Kinematic Systems. Materials 2021, 14, 7738. [Google Scholar] [CrossRef]
- Omrani, E.; Moghadam, A.D.; Menezes, P.L.; Rohatgi, P.K. Influences of graphite reinforcement on the tribological properties of self-lubricating aluminum matrix composites for green tribology, sustainability, and energy efficiency—A review. Int. J. Adv. Manuf. Technol. 2016, 83, 325–346. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.E.; Tichelaar, F.D.; Thompson, G.E.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochim. Acta 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, H.; Luo, P.; Luo, S.; Chen, J.; Kuang, Y. Preparation and characteristics of well-aligned macroporous films on aluminum by high voltage anodization in mixed acid. Surf. Coatings Technol. 2006, 201, 513–518. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W. Morphology and hydrophobicity of a polyurethane film molded on a porous anodic alumina template. Surf. Coatings Technol. 2006, 200, 3492–3495. [Google Scholar] [CrossRef]
- Korzekwa, J.; Gądek-Moszczak, A.; Bara, M. The Influence of Sample Preparation on SEM Measurements of Anodic Oxide Layers. Pract. Metallogr. 2016, 53, 36–49. [Google Scholar] [CrossRef]
- Sulka, G.D.; Zaraska, L.; Stępniowski, W.J. Anodic porous alumina as a template for nanofabrication. Encycl. Nanosci. Nanotechnol. 2011, 11, 261–349. [Google Scholar]
- Pietraszek, J.; Radek, N.; Goroshko, A.V.; Jana, A.; Ii, P. Challenges for the DOE methodology related to the introduction of Industry 4.0. Prod. Eng. Arch. 2020, 26, 190–194. [Google Scholar] [CrossRef]
- Dudek, A.; Lisiecka, B.; Radek, N.; Orman, Ł.J.; Pietraszek, J. Laser Surface Alloying of Sintered Stainless Steel. Materials 2022, 15, 6061. [Google Scholar] [CrossRef] [PubMed]
- Radek, N.; Pietraszek, J.; Gadek-Moszczak, A.; Orman, Ł.J.; Szczotok, A. The morphology and mechanical properties of ESD coatings before and after laser beam machining. Materials 2020, 13, 2331. [Google Scholar] [CrossRef] [PubMed]
- Korzekwa, J.; Bara, M.; Karpisz, D. Sample preparation methodology of the Al2O3 surface layers for self-lubricating sliding pair. In Proceedings of the 14th World Congress in Computational Mechanics (WCCM), ECCOMAS Congress 2020, Virtual, 11–15 January 2021; Volume 1000, pp. 1–11. [Google Scholar] [CrossRef]














| Current [A] | Time |
|---|---|
| 0.5 | 4 min 15 s |
| 0.4 | 5 min 19 s |
| 0.3 | 7 min 5 s |
| 0.2 | 10 min 37 s |
| 0.1 | 21 min 15 s |
| Variable | Load | Type of Electrolyte |
|---|---|---|
| Mass wear of TG15 | R = 0.87 | R = 0.43 |
| Friction coefficient | R = −0.63 | R = −0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzekwa, J.; Matczuk, R.; Hubkiewicz, K.; Bara, M.; Niedźwiedź, M.; Bochenek, D. Influence of Research Factors and Al2O3 Layer Production Parameters on Tribological and Microstructural Properties. Lubricants 2023, 11, 286. https://doi.org/10.3390/lubricants11070286
Korzekwa J, Matczuk R, Hubkiewicz K, Bara M, Niedźwiedź M, Bochenek D. Influence of Research Factors and Al2O3 Layer Production Parameters on Tribological and Microstructural Properties. Lubricants. 2023; 11(7):286. https://doi.org/10.3390/lubricants11070286
Chicago/Turabian StyleKorzekwa, Joanna, Robert Matczuk, Kinga Hubkiewicz, Marek Bara, Mateusz Niedźwiedź, and Dariusz Bochenek. 2023. "Influence of Research Factors and Al2O3 Layer Production Parameters on Tribological and Microstructural Properties" Lubricants 11, no. 7: 286. https://doi.org/10.3390/lubricants11070286
APA StyleKorzekwa, J., Matczuk, R., Hubkiewicz, K., Bara, M., Niedźwiedź, M., & Bochenek, D. (2023). Influence of Research Factors and Al2O3 Layer Production Parameters on Tribological and Microstructural Properties. Lubricants, 11(7), 286. https://doi.org/10.3390/lubricants11070286