Chemically Modifying Vegetable Oils to Prepare Green Lubricants
Abstract
:1. Introduction
- They are more expensive than mineral lubricants [4];
- They are relatively more toxic than vegetable oil-based lubricants;
- They are less readily biodegradable than vegetable oil-based lubricants;
- They have a lower friction tolerance and their exhausts pose problems in the environment;
- They do not work well with mineral oils.
2. Chemical and Physical Properties/Characteristics of Vegetable Oils
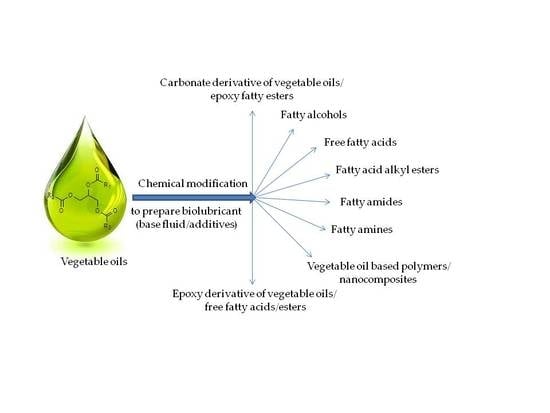
3. Chemical Modification/Derivatization of Vegetable Oils
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, P.; Das, M.; Upadhyay, M.; Das, T.; Mandal, A. Synthesis and Evaluation of Acrylate Polymers in Lubricating Oil. J. Chem. Eng. Data 2011, 56, 3752–3758. [Google Scholar] [CrossRef]
- Johnson, D.W.; Hils, J.E. Phosphate Esters, Thiophosphate Esters and Metal Thiophosphates as Lubricant Additives. Lubricants 2013, 1, 132–148. [Google Scholar] [CrossRef]
- Boyde, S. Green lubricants. Environmental benefits and impacts of lubrication. Green Chem. 2002, 4, 293–307. [Google Scholar] [CrossRef]
- Ripple, D.E.; Fuhrmann, J.F. Performance comparisons of synthetic and mineral oil crankcase lubricant base stocks. Lubr. Sci. 1989, 6, 209–232. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Yusop, R.M.; Salih, N. Synthesis, reactivity and application studies for different biolubricants. Chem. Cent. J. 2014, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, C.; Chao, M.; Wang, X. Natural Garlic Oil as a High-Performance, Environmentally Friendly, Extreme Pressure Additive in Lubricating Oils. ACS Sustain. Chem. Eng. 2014, 2, 798–803. [Google Scholar] [CrossRef]
- Ossia, C.V.; Han, H.G.; Kong, H. Additive properties of saturated very long chain fatty acids in castor and jojoba oils. J. Mech. Sci. Technol. 2008, 22, 1527–1536. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Adhvaryu, A.; Sharma, B.K. Poly(hydroxy thioether) Vegetable Oil Derivatives Useful as Lubricant Additives. U.S. Patent 7,279,448 B2, 9 October 2007. [Google Scholar]
- Erhan, S.Z.; Doll, K.M.; Sharma, B.K. Method of Making Fatty Acid Ester Derivatives. U.S. Patent 8,173,825 B2, 8 May 2012. [Google Scholar]
- Erhan, S.Z.; Doll, K.M.; Sharma, B.K. Method of Making Fatty Acid Ester Derivatives. U.S. Patent 20,080,154,053 A1, 26 June 2008. [Google Scholar]
- Doll, K.M.; Sharma, B.K.; Suarez, P.A. Process to Prepare a Phosphorous Containing Vegetable Oil Based Lubricant Additive. U.S. Patent 8,822,712 B1, 2 September 2014. [Google Scholar]
- Biswas, A.; Doll, K.M.; Cheng, H.N.; Sharma, B.K. Process for Preparation of Nitrogen-Containing Vegetable Oil-Based Lubricant Additive. U.S. Patent 8,841,470 B1, 23 September 2014. [Google Scholar]
- Heise, G.L.; Sharma, B.K.; Erhan, S.Z. Boron Containing Vegetable Oil Based Antiwear/Antifriction Additive and Their Preparation. U.S. Patent 9,156,859 B2, 13 October 2015. [Google Scholar]
- Erickson, F.L.; Anderson, R.E.; Landis, P.S. Vegetable Oil Derivatives as Lubricant Additives. U.S. Patent 5,282,989, 1 February 1994. [Google Scholar]
- Erickson, F.L.; Anderson, R.E.; Landis, P.S. Meadowfoam Oil and Meadowfoam Oil Derivatives as Lubricant Additives. U.S. Patent 4,925,581, 15 May 1990. [Google Scholar]
- Karmakar, G.; Ghosh, P. Soybean Oil as a Biocompatible Multifunctional Additive for Lubricating Oil. ACS Sustain. Chem. Eng. 2015, 3, 19–25. [Google Scholar] [CrossRef]
- Ghosh, P.; Karmakar, G. Evaluation of sunflower oil as a multifunctional lubricating oil additive. Int. J. Ind. Chem. 2014, 5. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P. Green Additives for Lubricating Oil. ACS Sustain. Chem. Eng. 2013, 1, 1364–1370. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M.; Karmakar, G. Castor oil as potential multifunctional additive in the formulation of eco-friendly lubricant. Polym. Bull. 2017. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Shahabuddin, M.; Hazrat, M.A.; Liaquat, A.M. Palm oil methyl ester and its emulsions effect on lubricant performance and engine components wear. Energy Procedia 2012, 14, 1748–1753. [Google Scholar] [CrossRef]
- Liu, Z.; Sharma, B.K.; Erhan, S.Z.; Biswas, A.; Wang, R.; Schuman, T.P. Oxidation and low temperature stability of polymerized soybean oil-based lubricants. Thermochim. Acta 2015, 601, 9–16. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Liu, Z.S.; Perez, J.M. Oxidation kinetic studies of unmodified and genetically modified vegetable oils using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Shashidhara, Y.M.; Jayaram, S.R. Vegetable oils as a potential cutting fluid—An evolution. Tribol. Int. 2010, 43, 1073–1081. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Rusto, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Ester hydroxy derivatives of methyl oleate: Tribological, oxidation and low temperature properties. Bioresour. Technol. 2008, 99, 7333–7340. [Google Scholar] [CrossRef] [PubMed]
- Borugadda, V.B.; Goud, V.V. Epoxidation of Castor Oil Fatty Acid Methyl Esters (COFAME) as a Lubricant base Stock Using Heterogeneous Ion-exchange Resin (IR-120) as a Catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Baye, T.; Becker, H.C. Exploration of vernonia galamensis in Ethiopia, and variation in fatty acid compositionof seed oil. Genet. Resour. Crop Evol. 2005, 52, 805–811. [Google Scholar] [CrossRef]
- Singh, R.K.; Padhi, S.K. Characterization of jatropha oil for the preparation of bio-diesel. Nat. Prod. Rad. 2009, 8, 127–132. [Google Scholar]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-Edible Plant Oils as New Sources for Biodiesel Production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Umarani, C.; Chinnusamy, T.R.; Krishnan, M.; Subramanian, R.; Neduzchezhain, N. Production and analysis of bio-diesel from non-edible oils—A review. Renew. Sustain. Energy Rev. 2009, 13, 825–834. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, S.; Prasad, R. Enhancement of viscosity index of mineral base oils. Indian J. Chem. Technol. 2006, 13, 398–403. [Google Scholar]
- Ghosh, P.; Hoque, M.; Karmakar, G.; Das, M.K. Dodecyl methacrylate and vinyl acetate copolymers as viscosity modifier and pour point depressant for lubricating oil. Int. J. Ind. Chem. 2017, 8, 197–205. [Google Scholar] [CrossRef]
- Heraud, A.; Pouligny, B. How Does a “Cloud Point” Diesel Fuel Additive Work? J. Colloid Interface Sci. 1992, 153, 378–391. [Google Scholar] [CrossRef]
- Sharma, B.K.; Stipanovic, A.J. Development of a new oxidation stability test method for lubricating oils using high-pressure differential scanning calorimetry. Thermochim. Acta 2003, 402, 1–18. [Google Scholar] [CrossRef]
- Ghosh, P.; Pantar, A.V.; Rao, U.S.; Sarma, A.S. Shear stability of polymers used as viscosity modifiers in lubricating oils. Indian J. Chem. Technol. 1998, 5, 309–314. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Hui, Y.H. Bailey’s Industrial Oil and Fats Products, Edible Oil and Fat Products: General Application, 5th ed.; Wiley: Blackwell, UK, 1995; Volume 1, pp. 19–44. [Google Scholar]
- Belgacem, M.N.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 39–66. [Google Scholar]
- Wang, Y.N.; Chen, M.H.; Ko, C.H.; Lu, P.J.; Chern, J.M.; Wu, C.H.; Chang, F.C. Lipase catalyzed transesterification of tung and palm oil for biodiesel. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011. [Google Scholar]
- Güner, F.S.; Yağcı, Y.; Erciyes, A.T. Polymers from triglyceride oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil—Biodegradable and potential base stoke for industrial lubricants. Ind. Crops Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Chhibber, V.K. Epoxidation in karanja oil for biolubricant applications. Int. J. Pharm. Biol. Sci. Arch. 2013, 1, 61–70. [Google Scholar]
- Meher, L.C.; Naik, S.N.; Das, L.M. Methanolysis of Pongamia pinnata (karanja) oil for production of biodiesel. J. Sci. Ind. Res. 2004, 63, 913–918. [Google Scholar]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and Composition of Jatropha Curcas Oil Seed from Malaysia and its Potential as Biodiesel Feedstock Feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Aigbodion, A.I.; Pillai, C.K.S. Preparation, analysis and applications of rubber seed oil and its derivatives in surface coatings. Prog. Org. Coat. 2000, 38, 187–192. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Betiku, E.; Ikhuomoregbe, D.I.O.; Ojumu, T.V. Production of biodiesel from crude neem oil feedstock and its emissions from internal combustion engines. Afr. J. Biotechnol. 2012, 11, 6178–6186. [Google Scholar] [CrossRef]
- Misra, R.D.; Murthy, M.S. Straight vegetable oils usage in a compression ignition engine: A review. Renew. Sustain. Energy Rev. 2010, 14, 3005–3013. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterisation of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Adnan, Q.; Ahmad, M.; Mehmood, A.; Farzana, K. Rheological studies and characterization of different oils. J. Chem. Soc. Pak. 2009, 2, 31. [Google Scholar]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef]
- Blin, J.; Brunschwig, C.; Chapuis, A.; Changotade, O.; Sidibe, S.; Noumi, E.; Girard, P. Characteristics of vegetable oils for use as fuel in stationary diesel engines-towards specifications for a standard in West Africa. Renew. Sustain. Energy Rev. 2013, 22, 580–597. [Google Scholar] [CrossRef] [Green Version]
- Harikrishnan, S.N.; Sabarish, R. Experimental Analysis of Direct Injection Diesel Engine Using Rubber Seed Oil. Middle-East J. Sci. Res. 2014, 20, 709–714. [Google Scholar]
- Bello, E.I.; Otu, F. Physicochemical Properties of Rubber (Hevea brasiliensis) Seed Oil, Its Biodiesel and Blends with Diesel. Br. J. Appl. Sci. Technol. 2015, 6, 261–275. [Google Scholar] [CrossRef]
- Padhi, S.K.; Singh, R.K. Optimization of esterification and transesterification of Mahua (Madhuca Indica) oil for production of biodiesel. J. Chem. Pharm. Res. 2010, 2, 599–608. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification-a review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Fabiano, B.; Reverberi, A.P.; Borghi, A.D.; Dovi, G. Biodiesel production via transesterification: Process safety insights from kinetic modeling. Theor. Found. Chem. Eng. 2012, 46, 673–680. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, X.; Tan, T. Preparation of a Water-Based Lubricant from Lignocellulosic Biomass and Its Tribological Properties. Ind. Eng. Chem. Res. 2017. [Google Scholar] [CrossRef]
- Schäffner, B.; Blug, M.; Kruse, D.; Polyakov, M.; Köckritz, A.; Martin, A.; Rajagopalan, P.; Bentrup, U.; Brückner, A.; Jung, S.; et al. Synthesis and Application of Carbonated Fatty Acid Esters from Carbon Dioxide Including a Life Cycle Analysis. ChemSusChem 2014, 7, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Kenar, J.A.; Knothe, G.; Copes, A.L. Synthesis and characterization of dialkyl carbonates prepared from mid-, long-chain, and guerbet alcohols. J. Am. Oil Chem. Soc. 2004, 81, 285–291. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galiá, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16. [Google Scholar] [CrossRef]
- Samarth, N.B.; Mahanwar, P.A. Modified Vegetable Oil Based Additives as a Future Polymeric Material—Review. Open J. Org. Polym. Mater. 2015, 5, 1–22. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.; Giordano, G.; Onida, B.; Cocina, D.; Tagarelli, A.; Giuffrè, A.M. Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid–base catalyst. Appl. Catal. A Gen. 2010, 378, 160–168. [Google Scholar] [CrossRef]
- Canakci, M.; Gerpen, J.V. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, C.A.C.; Ukogu, K.; Ibemesi, J.A. Catalysis in Biodiesel Production by Transesterification Processes-An Insight. J. Chem. 2010, 7, 1120–1132. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Wu, J.C.; Lau, S.K.; Cui, L.C.; Shimin, G.; Lim, A. Comparison of Novozym 435 and Amberlyst 15 as Heterogeneous Catalyst for Production of Biodiesel from Palm Fatty Acid Distillate. Energy Fuels 2009, 23, 1–4. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G., Jr.; Bruce, D.A. Transesterification of triacetin with methanol on Nafion® acid resins. J. Catal. 2007, 245, 381–391. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid superacid catalysis in fixed bed reactor under atmospheric pressure. Catal. Commun. 2004, 5, 721–723. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, B.S.; Kim, M.J.; Lee, K.Y. Development of heterogeneous catalyst system for esterification of free fatty acid contained in used vegetable oil. Stud. Surf. Sci. Catal. 2004, 153, 201–204. [Google Scholar]
- Chung, K.H.; Park, B.G. Esterification of oleic acid in soybean oil on zeolite catalysts with different acidity. J. Ind. Eng. Chem. 2009, 15, 388–392. [Google Scholar] [CrossRef]
- Peter, S.K.F.; Ganswindt, R.; Neuner, H.P.; Weidner, E. Alcoholysis of triacylglycerols by heterogeneous catalysis. Eur. J. Lipid Sci. Technol. 2002, 104, 324–330. [Google Scholar] [CrossRef]
- Sulek, M.W.; Kulczycki, A.; Malysa, A. Assessment of lubricity of compositions of fuel oil with biocomponents derived from rape-seed. Wear 2010, 268, 104–108. [Google Scholar] [CrossRef]
- Sukjit, E.; Dearn, K.D. Enhancing the lubricity of an environmentally friendly Swedish diesel fuel MK1. Wear 2011, 271, 1772–1777. [Google Scholar] [CrossRef]
- Maleque, M.A.; Masjuki, H.H.; Haseeb, A.S.M.A. Effect of mechanical factors on tribological properties of palm oil methyl ester blended lubricant. Wear 2000, 239, 117–125. [Google Scholar] [CrossRef]
- Masjuki, H.H.; Maleque, M.A. The effect of palm oil diesel fuel contaminated lubricant on sliding wear of cast irons against mild steel. Wear 1996, 198, 293–299. [Google Scholar] [CrossRef]
- Masjuki, H.H.; Maleque, M.A. Investigation of the anti-wear characteristics of palm oil methyl ester using a four-ball tribometer test. Wear 1997, 206, 179–186. [Google Scholar] [CrossRef]
- Malavolti, M.; Brandi, A.; Salvini, A.; Giomi, D. Transesterification of castor oil with trimethylchlorosilane: Simultaneous formation of fatty acid alkyl esters and α-monochlorohydrin. RSC Adv. 2015, 5, 77341–77347. [Google Scholar] [CrossRef]
- Madankar, C.S.; Pradhan, S.; Naik, S.N. Parametric study of reactive extraction of castor seed (Ricinus communis L.) for methyl ester production and its potential use as bio lubricant. Ind. Crops Prod. 2013, 43, 283–290. [Google Scholar] [CrossRef]
- Shi, Y.J.; Minami, I.; Grahn, M.; Bjorling, M.; Larsson, R. Boundary and elastohydrodynamic lubrication studies of glycerol aqueous solutions as green lubricants. Tribol. Int. 2014, 69, 39–45. [Google Scholar] [CrossRef]
- Langdon, W.K. Oxyalkylated Polyglycerols and Water-Based Lubricants Prepared Therefrom. U.S. Patent 4,265,774 A, 5 May 1981. [Google Scholar]
- Rudnick, L.R. Synthetics, Mineral Oils and Bio-Based Lubricants Chemistry and Technology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 362–386. ISBN 978-1-57444-723-1. [Google Scholar]
- Talukder, M.R.; Wu, J.C.; Chua, L.P.L. Conversion of Waste Cooking Oil to Biodiesel via Enzymatic Hydrolysis Followed by Chemical Esterification. Energy Fuels 2010, 24, 2016–2019. [Google Scholar] [CrossRef]
- Chowdhury, A.; Mitra, D.; Biswas, D. Biolubricant synthesis from waste cooking oil via enzymatic hydrolysis followed by chemical esterification. J. Chem. Technol. Biotechnol. 2013, 88, 139–144. [Google Scholar] [CrossRef]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; Giordano, R.L.C.; Tardioli, P.W. Lipase-Catalyzed Production of Biodiesel by Hydrolysis of Waste Cooking Oil Followed by Esterification of Free Fatty Acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Rathod, V.K. Ultrasound assisted enzyme catalyzed hydrolysis of waste cooking oil under solvent free condition. Ultrason. Sonochem. 2016, 32, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.; Pinto, A.F.; Gonçalves, A.G.; Mitchell, D.A.; Krieger, N. Biodiesel production from soybean soapstock acid oil by hydrolysis in subcritical water followed by lipase-catalyzed esterification using a fermented solid in a packed-bed reactor. Biochem. Eng. J. 2013, 81, 15–23. [Google Scholar] [CrossRef]
- Luo, H.; Xue, K.; Fan, W.; Li, C.; Nan, G.; Li, Z. Hydrolysis of Vegetable Oils to Fatty Acids Using Brønsted Acidic Ionic Liquids as Catalysts. Ind. Eng. Chem. Res. 2014, 53, 11653–11658. [Google Scholar] [CrossRef]
- Syaima, M.T.S.; Ong, K.H.; Noor, I.M.; Zamratul, M.I.M.; Brahima, S.A.; Hafizul, M.M. The synthesis of bio-lubricant based oil by hydrolysis and non-catalytic of palm oil mill effluent (POME) using lipase. Renew. Sustain. Energy Rev. 2015, 44, 669–675. [Google Scholar] [CrossRef]
- Biswas, A.; Sharma, B.K.; Willett, J.L.; Erhan, S.Z.; Cheng, H.N. Soybean oil as a renewable feedstock for nitrogen-containing derivatives. Energy Environ. Sci. 2008, 1, 639–644. [Google Scholar] [CrossRef]
- Yan, S.; Salley, S.O.; Simon, N.K.Y. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl. Catal. A Gen. 2009, 353, 203–212. [Google Scholar] [CrossRef]
- Fruth, A.; Strauss, J.; Stuhler, H. Process for the Preparation of Saturated Primary Fatty Amines by Hydrogenation of Unsaturated Fatty Acid Nitriles. U.S. Patent 5,175,370 A, 29 December 1992. [Google Scholar]
- Lundgren, S. Fatty Amine Salts as Friction Modifiers for Lubricants. Patent WO 2,015,059,162 A1, 30 April 2015. [Google Scholar]
- Lundgren, S. Fatty Amine Salts as Friction Modifiers for Lubricants. U.S. Patent 9,487,728 B2, 8 November 2016. [Google Scholar]
- Laufenberg, A.; Preibsch, W.; Schmitz, K.H. Lubricant Concentrate and an Aqueous Lubricant Solution Based on Fatty Amines, a Process for Its Production and Its Use. U.S. Patent 5,474,692 A, 12 December 1995. [Google Scholar]
- Pramanik, M.; Mendon, S.K.; Rawlins, J.W. Vegetable Oil Based Fatty Amide as Hydrophobes in Associative Thickener. J. Appl. Polym. Sci. 2013, 1530–1538. [Google Scholar] [CrossRef]
- Feairheller, S.H.; Bistline, R.G., Jr.; Bilyk, A.; Dudley, R.L.; Kozempel, M.F.; Haas, M.J. A novel technique for the preparation of secondary fatty amides. J. Am. Oil Chem. Soc. 1994, 71, 863–866. [Google Scholar] [CrossRef]
- Kopylov, L.I.; Shekhter, Y.N.; Gureev, A.A.; Bakaleinikov, M.B. Physicochemical and functional properties of mixtures of AKOR-1 inhibitor and alkanolamides. Chem. Technol. Fuels Oils 1980, 16, 62–64. [Google Scholar] [CrossRef]
- Kelley, M.J.; Robinson, E.A. Composition for Lubricating and Softening Textile Fibers. U.S. Patent 2,340,881, 8 February 1944. [Google Scholar]
- Gentry, D.R.; Stehlin, M.P.; Weers, J.J. Fatty Acid Amide Lubricity Aids and Related Methods for Improvement of Lubricity of Fuels. U.S. Patent 6,562,086 B1, 13 May 2003. [Google Scholar]
- Kammann, K.P., Jr. Modified Fatty Amides and Sulfurized Fatty Oils as Lubricant Additives. U.S. Patent 4921624 A, 1 May 1990. [Google Scholar]
- Sánchez, M.A.; Mazzieri, V.A.; Vicerich, M.A.; Vera, C.R.; Pieck, C.L. Influence of the Support Material on the Activity and Selectivity of Ru–Sn–B Catalysts for the Selective Hydrogenation of Methyl Oleate. Ind. Eng. Chem. Res. 2015, 54, 6845–6854. [Google Scholar] [CrossRef]
- Giraldo, L.; Camargo, G.; Tirano, J.; Moreno-Pirajan, J.C. Synthesis of Fatty Alcohol from Oil Palm Using a Catalyst of Ni-Cu Supported onto Zeolite. J. Chem. 2010, 7, 1138–1147. [Google Scholar] [CrossRef]
- Mueller, H.; Herold, C.P.; Tapavicza, S.V. Use of Selected Fatty Alcohols and Their Mixtures with Carboxylic Acid Esters as Lubricant Components in Water-Based Drilling Fluid Systems for Soil Exploration. U.S. Patent 6,716,799 B1, 6 April 2004. [Google Scholar]
- Cai, S.; Wang, L. Epoxidation of Unsaturated Fatty Acid Methyl Esters in the Presence of SO3H-functional Brønsted Acidic Ionic Liquid as Catalyst. Chin. J. Chem. Eng. 2011, 19, 57–63. [Google Scholar] [CrossRef]
- Abdullah, B.M.; Salimon, J. Epoxidation of Vegetable Oils and Fatty Acids: Catalysts, Methods and Advantages. J. Appl. Sci. 2010, 10, 1545–1553. [Google Scholar]
- Saithai, P.; Lecomte, J.; Dubreucq, E.; Tanrattanakul, V. Effects of different epoxidation methods of soybean oil on the characteristics of acrylated epoxidized soybean oil-co-poly(methyl methacrylate) copolymer. eXPRESS Polym. Lett. 2013, 7, 910–924. [Google Scholar] [CrossRef] [Green Version]
- Dinda, S.; Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Selective epoxidation of natural triglycerides using acidic ion exchange resin as catalyst. Asia-Pac. J. Chem. Eng. 2011, 6, 870–878. [Google Scholar] [CrossRef]
- Guidotti, M.; Gavrilova, E.; Galarneau, A.; Coq, B.; Psaro, R.; Ravasio, N. Epoxidation of methyl oleate with hydrogen peroxide. The use of Ti-containing silica solids as efficient heterogeneous catalysts. Green Chem. 2011, 13, 1806–1811. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Marchese, L.; Gallo, A.; Ravasio, N.; Psaro, R.; Guidotti, M. Epoxidation with hydrogen peroxide of unsaturated fatty acid methyl esters over Nb(V)-silica catalysts. Eur. J. Lipid Sci. Technol. 2013, 115, 86–93. [Google Scholar] [CrossRef]
- Somidi, A.K.R.; Sharma, R.V.; Dalai, A.K. Synthesis of Epoxidized Canola Oil Using a Sulfated-SnO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18668–18677. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Wang, J. Amphiphilic Polyoxometalate-Paired Polymer Coated Fe3O4: Magnetically Recyclable Catalyst for Epoxidation of Bio-Derived Olefins with H2O2. ACS Appl. Mater. Interfaces 2014, 6, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.B.; Katsiloulis, T. Calculation of Iodine Value from Measurements of Fatty Acid Methyl Esters of Some Oils: Comparison with the Relevant American Oil Chemists Society Method. J. Am. Oil Chem. Soc. 2000, 77, 1235–1238. [Google Scholar] [CrossRef]
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Schafer, V.; Kohler, R.; Pauli, A.; Fessenbecker, A. Corrosion Protection Additives Based on Epoxides. U.S. Patent 5,368,776 A, 29 November 1994. [Google Scholar]
- Rowland, R.G.; Migdal, C.A. Epoxidized Ester Additives for Reducing Lead Corrosion in Lubricants and Fuels. Patent EP 1805284A1, 11 July 2007. [Google Scholar]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B Environ. 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Erhan, S.Z. Synthesis of Hydroxy Thio-ether Derivatives of Vegetable Oil. J. Agric. Food Chem. 2006, 54, 9866–9872. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Adhvaryu, A.; Erhan, S.Z. Friction and wear behavior of thioether hydroxy vegetable oil. Tribol. Int. 2009, 42, 353–358. [Google Scholar] [CrossRef]
- Varshney, H.; Ahmad, A.; Rauf, A. Ring Opening of Epoxy Fatty Esters by Nucleophile to Form the Derivatives of Substituted β-Amino Alcohol. Food Nutr. Sci. 2013, 4, 21–24. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.M.; Erhan, S.Z. Synthesis of Carbonated Fatty Methyl Esters Using Supercritical Carbon Dioxide. J. Agric. Food Chem. 2005, 53, 9608–9614. [Google Scholar] [CrossRef] [PubMed]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Han, L.; Park, S.W.; Park, D.W. Silica grafted imidazolium-based ionic liquids: Efficient heterogeneous catalysts for chemical fixation of CO2 to a cyclic carbonate. Energy Environ. Sci. 2009, 2, 1286–1292. [Google Scholar] [CrossRef]
- Tamami, B.; Sohn, S.; Wilkes, G.L. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J. Appl. Polym. Sci. 2004, 92, 883–891. [Google Scholar] [CrossRef]
- Doll, K.M.; Erhan, S.Z. The improved synthesis of carbonated soybean oil using supercritical carbon dioxide at a reduced reaction time. Green Chem. 2005, 7, 849–854. [Google Scholar] [CrossRef]
- Tenhumberg, N.; Büttner, H.; Schäffner, B.; Kruse, D.; Blumensteinc, M.; Werner, T. Cooperative catalyst system for the synthesis of oleochemical cyclic carbonates from CO2 and renewable. Green Chem. 2016, 18, 3775–3788. [Google Scholar] [CrossRef]
- Polyakov, M.; Schäffner, B.; Kruse, D.; Martin, A.; Köckritz, A. Epoxide and cyclic carbonate with diisononyl succinate backbone as phthalate-free plasticizers. Tetrahedron Lett. 2016, 57, 964–968. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Arca, M.; Sharma, B.K.; Perez, J.M.; Doll, K.M. Gear oil formulation designed to meet bio-preferred criteria as well as give high performance. Int. J. Sustain. Eng. 2013, 6, 326–331. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P. Atom Transfer Radical Polymerization of Soybean Oil and Its Evaluation as a Biodegradable Multifunctional Additive in the Formulation of Eco-Friendly Lubricant. ACS Sustain. Chem. Eng. 2016, 4, 775–781. [Google Scholar] [CrossRef]
- Upadhyay, M.; Karmakar, G.; Kapur, G.S.; Ghosh, P. Multifunctional greener additives for lubricating oil. Polym. Eng. Sci. 2017. [Google Scholar] [CrossRef]
- Biresaw, G.; Asadauskas, S.J.; McClure, T.G. Polysulfide and biobased extreme pressure additive performance in vegetable vs paraffinic base oils. Ind. Eng. Chem. Res. 2012, 51, 262–273. [Google Scholar] [CrossRef]
- Landis, P.S. Telomerized Triglyceride Vegetable Oil for Lubricant Additives. U.S. Patent 5,229,023, 20 July 1993. [Google Scholar]
- Tian, Q.; Larock, R.C. Model studies and the ADMET polymerization of soybean oil. J. Am. Oil. Chem. Soc. 2002, 79, 479–488. [Google Scholar] [CrossRef]
- Rybak, A.; Fokou, P.A.; Meier, M.A.R. Metathesis as a versatile tool in oleochemistry. Eur. J. Lipid Sci. Technol. 2008, 110, 797–804. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Ring-opening metathesis polymerization of fatty acid derived monomers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5899–5906. [Google Scholar] [CrossRef]
- Mohan, N.; Natarajan, S.; KumareshBabu, S.P.; Siddaramaiah. Investigation on Sliding Wear Behaviour and Mechanical Properties of Jatropha Oil Cake-Filled Glass-Epoxy Composites. J. Am. Oil Chem. Soc. 2011, 88, 111–117. [Google Scholar] [CrossRef]







| Vegetable Oils | C12:0 | C14:0 | C16:0 | C18:0 | C16:1 | C18:1 | C18:2 | C18:3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Soybean oil | - | - | 11–12 | 3 | 0.2 | 24 | 53–55 | 6–7 | - |
| Sunflower oil | - | - | 7 | 5 | 0.3 | 20–25 | 63–68 | 0.2 | - |
| Rapeseed oil | - | - | 4–5 | 1–2 | 0.21 | 56–64 | 20–26 | 8–10 | 9.1 (20:1) |
| Palm oil | - | 1 | 37–41 | 3–6 | 0.4 | 40–45 | 8–10 | - | - |
| Rice bran oil | - | - | 20–22 | 2–3 | 0.19 | 42 | 31 | 1.1 | - |
| Cotton seed oil | - | 1 | 22–26 | 2–5 | 1.4 | 15–20 | 49–58 | - | - |
| Coconut oil | 44–52 | 13–19 | 8–11 | 1–3 | - | 5–8 | 0–1 | - | - |
| Corn (Maize) oil | - | - | 11–13 | 2–3 | 0.3 | 25–31 | 54–60 | 1 | - |
| Peanut/Ground nut | - | - | 10–11 | 2–3 | 0 | 48–50 | 39–40 | - | - |
| Sesame oil | - | - | 7–11 | 4–6 | 0.11 | 40–50 | 35–45 | - | - |
| Safflower oil | - | - | 5–7 | 1–4 | 0.08 | 13–21 | 73–79 | - | - |
| Karanja oil | - | - | 11–12 | 7–9 | - | 52 | 16–18 | - | - |
| Jatropha oil | - | 1.4 | 13–16 | 6–8 | - | 38–45 | 32–38 | - | - |
| Rubber seed oil | - | 2–3 | 10 | 9 | - | 25 | 40 | 16 | - |
| Mahua oil | - | - | 28 | 23 | - | 41–51 | 10–14 | - | - |
| Tung oil | - | - | 2.67 | 2.4 | - | 7.88 | 6.6 | 80.46 * | - |
| Neem oil | - | - | 18 | 18 | - | 45 | 18–20 | 0.5 | - |
| Castor oil | - | - | 0.5–1 | 0.5–1 | - | 4–5 | 2–4 | 0.5–1 | 83–85 # |
| Linseed oil | - | - | 4–5 | 2–4 | 0–0.5 | 19.1 | 12–18 | 56.6 | - |
| Olive oil | - | - | 13.7 | 2.5 | 1.8 | 71 | 10 | 0–1.5 | - |
| Vegetable Oils | Iodine Value | Pour Point (°C) | Cloud Point (°C) | Kinematic Viscosity at 40 °C (mm2/s) | Flash Point (°C) | Density at 15 °C (g/cm3) | Ref. |
|---|---|---|---|---|---|---|---|
| Soybean oil | 138–143 | −12 | −4 | 29 | 254 | 0.914 | [53,54,55] |
| Sunflower oil | 125–140 | −15 | −9.5 | 36 | 274 | 0.916 | [53,54,55,56] |
| Rapeseed oil | 98–105 | −15 | −2 | 35 | 246 | 0.912 | [53,54,55] |
| Palm oil | 48–58 | 23.6 | 25.2 | 39.4 | 252 | 0.919 | [57] |
| Rice bran oil | 103 | 13 | 16 | 38.2 | 184 | 0.906 | [41] |
| Cotton seed oil | 90–119 | −4.5 | −0.5 | 34 | 234 | 0.918 | [41,56] |
| Coconut oil | 8–11 | 12.7 | 13.1 | 27 | 266 | 0.918 | [53,54,55,56] |
| Peanut/Ground nut/Arachis oil | 84–100 | −7 | 4.5 | 40 | 271 | 0.903 | [53,54,55,58] |
| Sesame oil | 104–116 | −11 | −8 | 36 | 260 | 0.918 | [41,56,58] |
| Karanja oil | 81–90 | −4 | 2 | 38.8 | 212 | 0.9358 | [48,49] |
| Jatropha oil | 82–98 | −6 | 11 | 34 | 225 | 0.94 | [53,54,55] |
| Rubber seed oil | 104 | 18 | 25 | 33.89 | 228 | 0.928 | [59,60] |
| Mahua oil | 58–70 | 11 | 20 | 37.18 | 238 | 0.945 | [61] |
| Neem oil | 81 | 7 | 13 | 35.8 | 200 | 0.918 | [52] |
| Castor oil | 83–86 | −21 | −18 | 251 | 229 | 0.960 | [53,54,55,56] |
| Linseed oil | 168–204 | −15 | 5 | 26–29 | 241 | 0.938 | [41,58] |
| Safflower oil | 145 | −7 | −2 | 28.3 | 260 | 0.914 | [53,54,55] |
| Olive oil | 75–94 | −14 | −11 | 39 | 177 | 0.918 | [41,56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants 2017, 5, 44. https://doi.org/10.3390/lubricants5040044
Karmakar G, Ghosh P, Sharma BK. Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants. 2017; 5(4):44. https://doi.org/10.3390/lubricants5040044
Chicago/Turabian StyleKarmakar, Gobinda, Pranab Ghosh, and Brajendra K. Sharma. 2017. "Chemically Modifying Vegetable Oils to Prepare Green Lubricants" Lubricants 5, no. 4: 44. https://doi.org/10.3390/lubricants5040044
APA StyleKarmakar, G., Ghosh, P., & Sharma, B. K. (2017). Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants, 5(4), 44. https://doi.org/10.3390/lubricants5040044