Investigation of the Tribofilm Formation of HiPIMS Sputtered MoSx Thin Films in Different Environments by Raman Scattering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Deposition
2.2. Film Characterization
3. Results and Discussion
3.1. Structural and Mechanical Properties of the As-Deposited MoSx Film
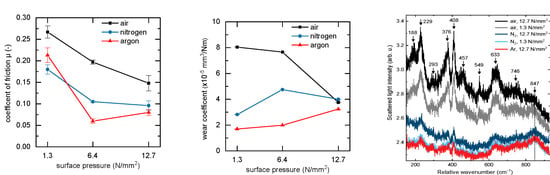
3.2. Tribological Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hütker, J.; Brümmer, A. Physics of a dry running unsynchronized twin screw expander. In Proceedings of the 8th International Conference on Compressors and Their Systems, London, UK, 9–10 September 2013; Elsevier: Amsterdam, The Netherlands, 2013; pp. 407–416. [Google Scholar]
- Debus, J.; Schindler, J.J.; Waldkirch, P.; Goeke, S.; Brümmer, A.; Biermann, D.; Bayer, M. Indication of worn WC/C surface locations of a dry-running twin-screw rotor by the oxygen incorporation in tungsten-related Raman modes. Appl. Phys. Lett. 2016, 109, 171601. [Google Scholar] [CrossRef]
- Gradt, T.; Schneider, T. Tribological Performance of MoS2 Coatings in Various Environments. Lubricants 2016, 4, 32. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. The Effects of Environmental Water and Oxygen on the Temperature-Dependent Friction of Sputtered Molybdenum Disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Vierneusel, B.; Schneider, T.; Tremmel, S.; Wartzack, S.; Gradt, T. Humidity resistant MoS2 coatings deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2013, 235, 97–107. [Google Scholar] [CrossRef]
- Colas, G.; Saulot, A.; Bouscharain, N.; Godeau, C.; Michel, Y.; Berthier, Y. How far does contamination help dry lubrication efficiency? Tribol. Int. 2013, 65, 177–189. [Google Scholar] [CrossRef]
- Singer, I.L.; Bolster, R.N.; Wegand, J.; Fayeulle, S.; Stupp, B.C. Hertzian stress contribution to low friction behavior of thin MoS2 coatings. Appl. Physic. Lett. 1990, 57, 995–997. [Google Scholar] [CrossRef]
- Meng, F.; Yang, C.; Han, H. Study on tribological performances of MoS2 coating at high temperature. J. Eng. Tribol. 2017, 232, 964–973. [Google Scholar] [CrossRef]
- Pope, L.E.; Panitz, J. The effects of Hertzian Stress and Test Atmosphere on the friction coefficients of MoS2 coatings. Surf. Coat. Technol. 1988, 36, 341–350. [Google Scholar] [CrossRef]
- Zhu, X.; Lauwerens, W.; Cosemans, P.; Van Stappen, M.; Celis, J.; Stals, L.; He, J. Different tribological behavior of MoS2 coatings under fretting and pinon-disk conditions. Surf. Coat. Technol. 2003, 163, 422–428. [Google Scholar] [CrossRef]
- Fleischauer, P. Fundamental aspects of the electronic structure, materials properties and lubrication performance of sputtered MoS2 films. Thin Solid Film 1987, 154, 309–322. [Google Scholar] [CrossRef]
- Windischmann, H. Intrinsic stress in sputter-deposited thin films. Crit. Rev. Solid State Mater. Sci. 1992, 17, 547–596. [Google Scholar] [CrossRef]
- Davis, C.A. A simple model for the formation of compressive stress in thin films by ion bombardment. Thin Solid Films 1993, 226, 30–34. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Laukkanen, A.; Wallin, K.; Hogmark, S.; Jacobson, S.; Wiklund, U.; Souza, R.M.; Ståhle, P. Residual stresses in TiN, DLC and MoS2 coated surfaces with regard to their tribological fracture behaviour. Wear 2009, 267, 2142–2156. [Google Scholar] [CrossRef]
- Wieting, T.J. Long-Wavelength lattice vibrations of MoS2 and GaSe. Solid State Commun. 1973, 12, 931–935. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91. [Google Scholar] [CrossRef]
- Stacy, A.M.; Hodul, D.T. Raman spectra of IVB and VIB transition metal disulfides using laser energies near the absorption edges. J. Phys. Chem. Solids 1985, 46, 405–409. [Google Scholar] [CrossRef]
- Moldenhauer, H. Resonant Raman Scattering Characterizes Thermally Annealed HiPIMS Deposited. Surf. Coat. Technol. 2019, 377, 124891. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Zhang, X. Effect of crystallographic orientation on fretting wear behaviour of MoS coatings in dry and humid air. Thin Solid Film 2001, 396, 69–77. [Google Scholar] [CrossRef]
- Holinski, R.; Gänsheimer, J. A study of the lubricating mechanism of molybdenum disulfide. Wear 1972, 19, 329–342. [Google Scholar] [CrossRef]
- Matsumoto, K. Tribological Performance of Sputtered MoS2 Films in Various Environment—Influence of oxygen concentration, water vapor and gas species. In Proceedings of the 8th European Space Mechanisms & Tribology Symposium, ESA SP-438, Toulouse, France, 29 September–1 October 1999. [Google Scholar]
- Colas, G.; Saulot, A.; Regis, E.; Berthier, Y. Investigation of crystalline and amorphous MoS2 based coatings: Towards developing new coatings for space applications. Wear 2015, 330, 448–460. [Google Scholar] [CrossRef]
- Colbert, R.S.; Sawyer, W.G. Thermal dependence of the wear of molybdenum disulphide coatings. Wear 2010, 269, 719–723. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. Surface and Subsurface Contributions of Oxidation and Moisture to Room Temperature Friction of Molybdenum Disulfide. Tribol. Lett. 2013, 53, 329–336. [Google Scholar] [CrossRef]
- Moldenhauer, H.; Bayer, M.; Debus, J.; Nikolov, A.; Brümmer, A. Raman scattering study of micrometer-sized spots of magnetite and hematite formed at 18CrNiMo7-6 screw rotor surfaces due to liquid-free, unsynchronized operation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 425, 12016. [Google Scholar] [CrossRef]
- Mo, T.; Xu, J.; Yang, Y.; Li, Y. Effect of carburization protocols on molybdenum carbide synthesis and study on its performance in CO hydrogenation. Catal. Today 2016, 261, 101–115. [Google Scholar] [CrossRef]
- Chen, J.M.; Wang, C.S. Second order Raman spectrum of MoS2. Solid State Commun. 1974, 14, 857–860. [Google Scholar] [CrossRef]
- Qiu, J. Formation of N-doped molybdenum carbide confined in hierarchical and hollow carbon nitride microspheres with enhanced sodium storage properties. J. Mater. Chem. 2016, 4, 13296–13306. [Google Scholar] [CrossRef]
- Lavik, M.T.; Medved, T.M.; Moore, G.D. Oxidation characteristics of MoS2 and other solid lubricants. ASLE Trans. 1968, 11, 44–55. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zhang, Y.; Xiao, J.; Yu, L.; Liu, Q.; Ning, Y.; Zhou, Z.; Chen, H.; Huang, W.; et al. Enhanced oxidation resistance of active nanostructures via dynamic size effect. Nat. Commun. 2017, 8, 14459. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, J.; Wang, K.; Shen, M.; Zeng, S.; Dayeh, S.A.; Feng, L.; Xiang, B. Lattice strain effects on the optical properties of MoS2 nanosheets. Sci. Rep. 2014, 4, 5649. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D. Influence of the deposition parameters on the texture and mechanical properties of magnetron sputterd cubic MoNx thin films. Materialia 2018, 5, 100186. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, Z.; Chen, K.J. In Situ Resonant Raman Spectroscopy to Monitor the Surface Functionalization of MoS2 and WSe2 for High-k Integration: A First-Principles Study. Langmuir 2018, 34, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lauwerens, W.; He, J.; Celis, J.-P. Reorientation of randomly oriented MoSx coatings during fretting wear tests. Tribol. Lett. 2004, 17, 607–612. [Google Scholar] [CrossRef]
- Fleischauer, P. Effects of Crystallite Orientation on Environmental Stability and Lubrication Properties of SputteredMoS2 Thin Films. ASLE Trans. 2008, 27, 82–88. [Google Scholar] [CrossRef]
- Singer, I.L. Role of third bodies in friction and wear of protective coatings. J. Vac. Sci. Technol. 2003, A21, 232–241. [Google Scholar] [CrossRef]
- Singer, I.L.; Fayeulle, S.; Ehni, P.D. Wear behavior of triode-sputtered MoS 2 coatings in dry sliding contact with steel and ceramics. Wear 1996, 195, 7–20. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillmann, W.; Wittig, A.; Stangier, D.; Thomann, C.-A.; Moldenhauer, H.; Debus, J.; Aurich, D.; Brümmer, A. Investigation of the Tribofilm Formation of HiPIMS Sputtered MoSx Thin Films in Different Environments by Raman Scattering. Lubricants 2019, 7, 100. https://doi.org/10.3390/lubricants7110100
Tillmann W, Wittig A, Stangier D, Thomann C-A, Moldenhauer H, Debus J, Aurich D, Brümmer A. Investigation of the Tribofilm Formation of HiPIMS Sputtered MoSx Thin Films in Different Environments by Raman Scattering. Lubricants. 2019; 7(11):100. https://doi.org/10.3390/lubricants7110100
Chicago/Turabian StyleTillmann, Wolfgang, Alexandra Wittig, Dominic Stangier, Carl-Arne Thomann, Henning Moldenhauer, Jörg Debus, Daniel Aurich, and Andreas Brümmer. 2019. "Investigation of the Tribofilm Formation of HiPIMS Sputtered MoSx Thin Films in Different Environments by Raman Scattering" Lubricants 7, no. 11: 100. https://doi.org/10.3390/lubricants7110100
APA StyleTillmann, W., Wittig, A., Stangier, D., Thomann, C. -A., Moldenhauer, H., Debus, J., Aurich, D., & Brümmer, A. (2019). Investigation of the Tribofilm Formation of HiPIMS Sputtered MoSx Thin Films in Different Environments by Raman Scattering. Lubricants, 7(11), 100. https://doi.org/10.3390/lubricants7110100