Ionic Liquid Crystals in Tribology
Abstract
:1. Introduction
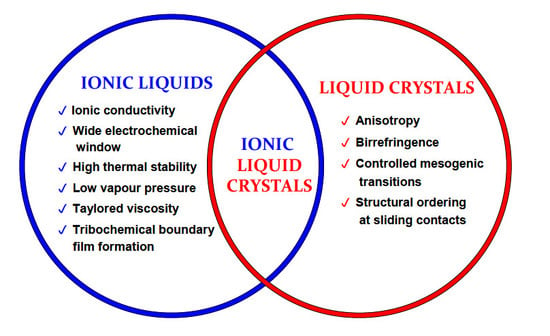
2. Ionic Liquid Crystals
2.1. Ionic Liquid Crystal Lubricants
2.1.1. Chain Length
2.1.2. Fatty Acids and Other Combinations and Synergistic Effects
2.1.3. Rheology
2.2. Sliding Configurations and Applications
3. Conclusions
Funding
Conflicts of Interest
References
- Mitov, M. Liquid-crystal science from 1888 to 1922: Building a revolution. ChemPhysChem 2014, 15, 1245–1250. [Google Scholar] [CrossRef]
- Goosens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic liquid crystals: Versatile materials. Chem. Rev. 2016, 8, 4643–4807. [Google Scholar] [CrossRef]
- Carrión, F.J.; Martínez-Nicolás, G.; Iglesias, P.; Sanes, J.; Bermúdez, M.D. Liquid crystals in tribology. Int. J. Mol. Sci. 2009, 10, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behaviour of biolubricant basestocks and additives. Renew. Sustain. Energy Rev. 2008, 93, 145–147. [Google Scholar] [CrossRef]
- Gong, X.; Li, L. Nanometer-thick ionic liquids as boundary lubricants. Adv. Eng. Mater. 2018, 20, 1700617. [Google Scholar] [CrossRef]
- Iglesias, P.; Bermúdez, M.D.; Carrión, F.J.; Martínez-Nicolás, G. Friction and wear of aluminium-steel contacts lubricated with ordered fluids-neutral and ionic liquid crystals as oil additives. Wear 2004, 256, 386–392. [Google Scholar] [CrossRef]
- Axenov, K.V.; Laschat, S. Thermotropic liquid crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef] [Green Version]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4208. [Google Scholar] [CrossRef]
- Gao, Y.M.; Jiang, Y.; Hu, W.J.; Jiang, H.Z.; Li, J.S. Cholesteryl liquid crystals as oil-based lubricant additives: Effect of mesogenic phases and structures on tribological characteristics. Langmuir 2019, 35, 6981–6992. [Google Scholar] [CrossRef]
- Tariq, M.; Serro, A.P.; Colaco, R.; Saramago, B.; Lopes, J.N.C.; Rebelo, L.P.N. Effect of alkyl chain length on the adsorption and frictional behviour of 1-alkyl-3-methylimidazolium chloride ionic liquid surfactants on gold surfaces. Coll. Surf. A. Physicochem. Eng. Asp. 2011, 377, 361–366. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Prado, S.; Domínguez-Pérez, M.; Rilo, E.; García-Garabal, S.; Segado, L.; Franjo, C.; Cabeza, O. Experimental measurement of the hygroscopic grade on eight imidazolium based ionic liquids. Fluid Phase Equil. 2009, 278, 36–40. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Complex fluids in tribology to reduce friction: Mesogenic fluids, ionic liquids and ionic liquid crystals. Tribol. Int. 2013, 65, 3–12. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Amann, T.; Dold, C.; Metwalli, E.; Müller-Buschbaum, P.; Kailer, A.; Friedrich, C. Triborheology and orientational dynamics of ionic liquid crystals. J. Mol. Liq. 2014, 192, 118–126. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yu, L.; Zhang, P.; Zhang, Z.; Wu, Z. Tribological behavior of 1-methyl-3-hexadecylimidazolium tetrafluoroborate ionic liquid crystal as a neat lubricant and as an additive of liquid paraffin. Tribol. Lett. 2012, 46, 49–54. [Google Scholar] [CrossRef]
- Maximo, G.J.; Sanos, R.J.B.N.; Lopes, J.A.; Costa, M.C.; Meirelles, A.J.A.; Coutinho, J.A.P. Lipidic protic ionic liquid crystals. ACS Chem. Eng. 2014, 2, 672–682. [Google Scholar] [CrossRef]
- Tadokoro, C.; Araya, S.; Okubo, H.; Nakano, K. Polarization observation of adsorption behaviour of fatty acids using optical anisotropy of liquid crystal. Tribol. Lett. 2016, 64, 30. [Google Scholar] [CrossRef]
- Toledo, A.A.C.; Maximo, G.J.; Costa, M.C.; Cunha, R.L.; Pereira, J.F.B.; Kurnia, K.A.; Batista, E.A.C.; Meirelles, A.J.A. Phase behaviour and physical properties of new biobased ionic liquid crystals. J. Phys. Chem. B 2017, 121, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Khatri, O.P. Fatty acid ionic liquids as environmentally friendly lubricants for low friction and wear. RSC Adv. 2016, 6, 3462–3469. [Google Scholar] [CrossRef]
- Santos, D.; Costa, F.; Franceschi, E.; Santos, A.; Dariva, C.; Mattedi, S. Synthesis and physico-chemical properties of two protic ionic liquids based on stearate anion. Fluid Phase Equilibria 2014, 378, 132–140. [Google Scholar] [CrossRef]
- Álvarez, S.; Mattedi, M.; Martín-Pastor, M.; Aznar, M.; Iglesias, M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluid Phase Equilibria 2010, 299, 354–366. [Google Scholar] [CrossRef]
- Shi, Y.; Larsson, R. Non-corrosive and biomaterials protic ionic liquids with high lubricating performance. Tribol. Lett. 2016, 63, 1. [Google Scholar] [CrossRef]
- Avilés, M.D.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Effects of protic ionic liquid crystal additives on the water lubricated sliding wear and friction of sapphire against stainless steel. Wear 2018, 408, 56–64. [Google Scholar] [CrossRef]
- Carrión, F.J.; Avilés, M.D.; Nakano, K.; Tadokoro, C.; Nagamine, T.; Bermúdez, M.D. Diprotic ammonium palmitate ionic liquid crystal and nanodiamonds in aqueous lubrication. Film thickness and influence of sliding speed. Wear 2019, 418, 241–252. [Google Scholar] [CrossRef]
- Chen, M.; Liu, B.; Wang, X.; Fu, Y.; Hao, J.; Li, H. Zero-charged catanionic lamellar liquid crystals doped with fullerene C60 potential applications in tribology. Soft Matter 2017, 13, 6250–6258. [Google Scholar] [CrossRef]
- Tadokoro, C.; Araya, S.; Watanabe, M.; Okubo, H.; Sasaki, S. Synergy of two fatty acids as additives on lubricity of a nematic liquid crystal 5CB. Lubr. Sci. 2018, 30, 83–90. [Google Scholar] [CrossRef]
- Lu, R.; Mori, S.; Tani, H.; Tagawa, N.; Koganezawa, S. Low friction properties of associated carboxylic acids induced by molecular orientation. Tribol. Int. 2017, 113, 36–42. [Google Scholar] [CrossRef]
- Chen, L.; Ge, L.; Fan, L.; Guo, R. Microstructure and tribological properties of lamellar liquid crystals formed by ionic liquids as co-surfactants. Langmuir 2019, 35, 4037–4045. [Google Scholar] [CrossRef]
- Chen, L.; Han, J.; Ge, L.; Fan, L.; Guo, R. Improvement in lubricating properties of TritonX-100/n-C10H21OH/H2O lamellar liquid crystals with the amphiphilic ionic liquid 1-alkyl-3-methylimidazolium hexafluorophosphate. J. Coll. Interface Sci. 2018, 522, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Q.; Liu, P.; Hao, J.C. Polyoxometalate-lyotropic liquid crystal hybrid material in room-temperature ionic liquids. J. Nanosci. Nanotechnol. 2011, 11, 2168–2174. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, b.; Liu, W.; Hao, J. Carbon nanotubes incorporated within lyotropic hexagonal liquid crystal formed in room-temperature ionic liquids. Langmuir 2007, 23, 8549–8553. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Ge, L.L.; Guo, R. Microstructure of lamellar liquid crystal in Tween 85/[Bmim]PF6/H2O system and applications as Ag nanoparticle synthesis and lubrication. J. Mater. Res. 2009, 24, 333–341. [Google Scholar] [CrossRef]
- Madhavi, A.B.; Sastry, S.S. Rheological properties of cholesteric liquid crystals as lubricant additives. Int. J. Modern Phys. B 2019, 33, 1950014. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Rheological characterization of ionic liquid crystals with promising tribological performance. Soft Matter 2012, 8, 9840–9846. [Google Scholar] [CrossRef]
- Amann, T.; Kailer, A.; Beyer, S.; Stehr, W.; Metzger, B. Development of sintered bearings with minimal friciton losses and maximum lifetime using infiltrated liquid crystalline lubricants. Tribol. Int. 2016, 98, 282–291. [Google Scholar] [CrossRef]
- Moksin, V.; Kilikevicius, A.; Vekteris, V. Investigation of dissipative properties of liquid crystalline lubricant layer. J. Vibroeng. 2012, 14, 502–508. [Google Scholar]
- Amann, T.; Kailer, A. Analysis of the ultralow friction behaviour of a mesogenic fluid in a reciprocating contact. Wear 2011, 271, 1701–1706. [Google Scholar] [CrossRef]
- Elemsimit, H.A.; Grecov, D. Impact of liquid crystal additives on a canola oil-based bio-lubricant. Part J J. Eng. Tribol. 2015, 229, 126–135. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés, M.D.; Sánchez, C.; Pamies, R.; Sanes, J.; Bermúdez, M.D. Ionic Liquid Crystals in Tribology. Lubricants 2019, 7, 72. https://doi.org/10.3390/lubricants7090072
Avilés MD, Sánchez C, Pamies R, Sanes J, Bermúdez MD. Ionic Liquid Crystals in Tribology. Lubricants. 2019; 7(9):72. https://doi.org/10.3390/lubricants7090072
Chicago/Turabian StyleAvilés, M.D., C. Sánchez, R. Pamies, J. Sanes, and M.D. Bermúdez. 2019. "Ionic Liquid Crystals in Tribology" Lubricants 7, no. 9: 72. https://doi.org/10.3390/lubricants7090072
APA StyleAvilés, M. D., Sánchez, C., Pamies, R., Sanes, J., & Bermúdez, M. D. (2019). Ionic Liquid Crystals in Tribology. Lubricants, 7(9), 72. https://doi.org/10.3390/lubricants7090072