Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook
Abstract
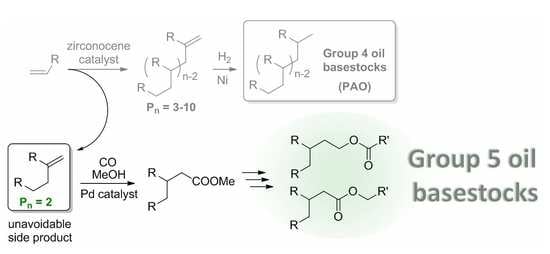
:1. Introduction
2. Materials and Methods
2.1. General Experimental Remarks
2.2. Methoxycarbonylation of Methylenealkanes
2.3. Synthesis of Branched Alcohols and Esters E2–E5
2.3.1. 3-Butylnonan-1-ol
2.3.2. 3-Hexylundecan-1-ol
2.3.3. 3-Butylnonyl 2-ethylhexanoate (E2)
2.3.4. 3-Hexylundecyl 2-ethylhexanoate (E3)
2.3.5. Octyl 3-hexylundecanoate (E4)
2.3.6. 2-Ethylhexyl 3-hexylundecanoate (E5)
2.4. Viscosity Measurements
3. Results
3.1. Synthesis of Branched Esters
3.2. Viscosity of Branched Esters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atabani, A.E.; Badruddin, I.A.; Mekhilef, S.; Silitonga, A.S. A review on global fuel economy standards, labels and technologies in the transportation sector. Renew. Sustain. Energy Rev. 2011, 15, 4586–4610. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhao, Y.; Ma, K.; Wang, Q. Friction behavior and wear protection ability of selected base lubricants. Friction 2016, 4, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Hope, K. PAO Contributions to Energy Efficiency in 0W-20 Passenger Car Engine Oils. Lubricants 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Maroto-Centeno, J.-A.; Pérez-Gutiérrez, T.; Fernández-Ruíz-Morón, L.; Quesada-Pérez, M. Prediction of fuel economy performance of engine lubricants based on laboratory bench tests. Tribol. Int. 2016, 94, 67–70. [Google Scholar] [CrossRef]
- Ishizaki, K.; Nakano, M. Reduction of CO2 Emissions and Cost Analysis of Ultra-Low Viscosity Engine Oil. Lubricants 2018, 6, 102. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Rao, P.V.C.; Choudary, N.V. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubric. Sci. 2012, 24, 23–44. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Vinu, R. Characterization of Thermal Stability of Synthetic and Semi-Synthetic Engine Oils. Lubricants 2015, 3, 54–79. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.K.; McAuley, K.B.; Peppley, B.A. Biolubricants through renewable hydrocarbons: A perspective for new opportunities. Renew. Sustain. Energy Rev. 2019, 113, 109261. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Kodali, D.R. High performance ester lubricants from natural oils. Ind. Lubr. Tribol. 2002, 54, 165–170. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food. Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel Strategies for the Production of Fuels, Lubricants, and Chemicals from Biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Kannan, K.T.; Rameshbabu, S. Tribological properties of modified jojoba oil as probable base stoke of engine lubricant. J. Mech. Sci. Technol. 2018, 32, 1739–1747. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Mohamad, E.N.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Mannekote, J.K.; Kailas, S.V.; Venkatesh, K.; Kathyayini, N. Environmentally friendly functional fluids from renewable and sustainable sources-A review. Renew. Sustain. Energy Rev. 2018, 81, 1787–1801. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Chan, C.-H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Bell, A.T.; Toste, F.D. Synthesis of Biomass-Derived Ethers for Use as Fuels and Lubricants. ChemSusChem 2019, 12, 2835–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kioupis, L.I.; Maginn, E.J. Molecular Simulation of Poly-α-olefin Synthetic Lubricants? Impact of Molecular Architecture on Performance Properties. J. Phys. Chem. B 1999, 103, 10781–10790. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J. Bio Tribo Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- DiMaio, A.J. Process for the Oligmerization of Alpha-Olefins Having Low. Unsaturation. Patent US7129306, 31 November 2006. [Google Scholar]
- Patil, A.O.; Bodige, S.; Luo, S.; Chu, J.W.; Stavens, K.; Harrrington, B.A. Ultra High Viscosity Synthetic Base Stocks and Process for Preparing Same. U.S. Patent Application US2014213834, 31 July 2014. [Google Scholar]
- Small, B.L.; Hope, K.D.; Masino, A.P.; McDaniel, M.P.; Buck, R.M.; Beaulieu, W.B.; Yang, Q.; Baralt, E.J.; Netemeyer, E.J.; Kreischer, B. Oligomerization of Alpha Olefins Using Metallocene-SSA Catalyst Systems and Use of the Resultant Polyalphaolefins to Prepare Lubricant Blends. Patent US8536391, 17 September 2013. [Google Scholar]
- Hagemeister, M.P.; Jiang, P.; Wu, M.M.; Yang, N. Production of Shear-Stable High Viscosity PAO. Patent US9365663, 14 June 2016. [Google Scholar]
- Wu, M.M.; Coker, C.L.; Walzer, J.F.; Jiang, P.; Rucker, S.P. Process to Produce High Viscosity Fluids. Patent US7989670, 2 August 2011. [Google Scholar]
- Park, J.H.; Jang, Y.E.; Jeon, J.Y.; Go, M.J.; Lee, J.; Kim, S.K.; Lee, S.-I.; Lee, B.Y. Preparation of ansa-metallocenes for production of poly(α-olefin) lubricants. Dalton Trans. 2014, 43, 10132–10138. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P. Alkyl-substituted cyclopentadienyl- and phospholyl-zirconium/MAO catalysts for propene and 1-hexene oligomerization. J. Mol. Catal. A Chem. 2002, 180, 43–58. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P.; Krüger, R.-P.; Hanselmann, R. Analyses of Propene and 1-Hexene Oligomers from Zirconocene/MAO Catalysts—Mechanistic Implications by NMR, SEC, and MALDI-TOF MS. Macromol. Chem. Phys. 2002, 203, 129–138. [Google Scholar] [CrossRef]
- Fujikawa, S.; Yokota, K.; Okano, M.; Tsuji, M. Method for Producing α-Olefin Oligomers and Lubricating Oil Compositions. Patent Application US2011207977, 25 July 2011. [Google Scholar]
- Welle, A.; Wassenaar, J.; Slawinski, M. Use of a Metallocene Catalyst to Produce a Polyalpha-Olefin. Patent US9688792, 27 June 2017. [Google Scholar]
- Wu, M.M.; Coker, C.L.; Walzer, J.F.; Jiang, P. Process to Produce Low Viscosity Poly-Alfa-Olefins. Patent US8207390, 26 June 2012. [Google Scholar]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene ‘dimer’. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Gabriëls, D.; Hernández, W.Y.; Sels, B.; Van Der Voort, P.; Verberckmoes, A. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal. Sci. Technol. 2015, 5, 3876–3902. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G. Characterization of esters of fatty acids and dicarboxylic acids with Guerbet alcohols. J. Am. Oil Chem. Soc. 2001, 78, 537–540. [Google Scholar] [CrossRef]
- Waykole, C.; Bhowmick, D.N.; Pratap, A. Synthetic Base Stock Based on Guerbet Alcohols. J. Am. Oil Chem. Soc. 2014, 91, 1407–1416. [Google Scholar] [CrossRef]
- Matsu-ura, T.; Sakaguchi, S.; Obora, Y.; Ishii, Y. Guerbet Reaction of Primary Alcohols Leading to β-Alkylated Dimer Alcohols Catalyzed by Iridium Complexes. J. Org. Chem. 2006, 71, 8306–8308. [Google Scholar] [CrossRef] [PubMed]
- Goulas, K.A.; Sreekumar, S.; Song, Y.; Kharidehal, P.; Gunbas, G.; Dietrich, P.J.; Johnson, G.R.; Wang, Y.C.; Grippo, A.M.; Grabow, L.C.; et al. Synergistic Effects in Bimetallic Palladium–Copper Catalysts Improve Selectivity in Oxygenate Coupling Reactions. J. Am. Chem. Soc. 2016, 138, 6805–6812. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.K.; Oumar-Mahamat, H.; Cheng, H.; Blain, D.A.; Cooper, K.K.; Carey, J.T.; Douglass, M.R.; Kanga, P.R.; Patil, A.O.; Bodige, S.; et al. Low Viscosity Low Volatility Lubricating Oil Base Stocks and Methods of Use Thereof. Patent US10077409, 18 September 2018. [Google Scholar]
- Patil, A.; Lewis, K.G.; Bodige, S.; Zushma, S. Ester Compounds, Lubricating Oil Composition Containing Same and Process for Making Same. Patent Application US2019062663, 28 February 2019. [Google Scholar]
- Sato, H.; Kashiwamura, T.; Okamoto, T.; Yokota, K. Carbonyl Compound Containing Long-Chain Branched Alkyl Group. Patent US7402610, 22 July 2008. [Google Scholar]
- Nafant’ev, I.E.; Sevostyanova, N.T.; Batashev, S.A.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Ivchenko, P.V. Synthesis of methyl β-alkylcarboxylates by Pd/diphosphine-catalyzed methoxycarbonylation of methylenealkanes RCH2CH2C(R)=CH2. Appl. Catal. A Gen. 2019, 581, 123–132. [Google Scholar] [CrossRef]
- Hayashi, T.; Kawabata, Y.; Isoyama, T.; Ogata, I. Platinum Chloride–Diphosphine–Tin(II) Halide Systems as Active and Selective Hydroformylation Catalysts. Bull. Chem. Soc. Jpn. 1981, 54, 3438–3446. [Google Scholar] [CrossRef] [Green Version]
- Sulzbacher, M.; Bergmann, E. Synthesis of p-alkylstyrenes. J. Org. Chem. 1948, 13, 303–308. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Dienes, G.J. Activation Energy for Viscous Flow and Short-Range Order. J. Appl. Phys. 1953, 24, 779–782. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Arinina, M.P.; Polyakova, M.Y.; Kulichikhin, V.G.; Malkin, A.Y. Rheological comparison of light and heavy crude oils. Fuel 2016, 186, 157–167. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Strelets, L.A. Basic Fundamentals of Petroleum Rheology and Their Application for the Investigation of Crude Oils of Different Natures. Energy Fuels 2018, 32, 268–278. [Google Scholar] [CrossRef]




| Oil Basestock | Dynamic Viscosity, at T (°C), mPa·s | Pour Point, °C | Ea, 1 kJ/mol | E’a, 2 kJ/mol | |||||
|---|---|---|---|---|---|---|---|---|---|
| −40 | 25 | 40 | 100 | ||||||
| E1 | 267 | 5.86 | 3.75 | 1.38 | −58 | 15.7 | 17.5 | −116.3 | 0.076 |
| E2 | 392 | 5.93 | 3.67 | 1.25 | −56 | 17.6 | 18.4 | −114.2 | 0.056 |
| E3 | 893 | 11.5 | 6.28 | 1.73 | −60 | 20.7 | 20.3 | −130.4 | 0.027 |
| E4 | 910 | 12.4 | 7.16 | 1.88 | −53 | 21.4 | 20.1 | −134.2 | 0.029 |
| E5 | 852 | 10.9 | 6.41 | 1.83 | −60 | 20.3 | 19.2 | −125.7 | 0.045 |
| P1 | 725 | 10.7 | 6.57 | 1.75 | −62 | 19.1 | 19.6 | −132.6 | 0.034 |
| P2 | 1850 | 19.2 | 11.7 | 2.67 | −57 | 21.4 | 20.0 | −129.8 | 0.041 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Bagrov, V.; Vinogradov, A.; Vinogradov, A.; Ilyin, S.; Sevostyanova, N.; Batashev, S.; Ivchenko, P. Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants 2020, 8, 50. https://doi.org/10.3390/lubricants8050050
Nifant’ev I, Bagrov V, Vinogradov A, Vinogradov A, Ilyin S, Sevostyanova N, Batashev S, Ivchenko P. Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants. 2020; 8(5):50. https://doi.org/10.3390/lubricants8050050
Chicago/Turabian StyleNifant’ev, Ilya, Vladimir Bagrov, Alexander Vinogradov, Alexey Vinogradov, Sergey Ilyin, Nadezhda Sevostyanova, Sergey Batashev, and Pavel Ivchenko. 2020. "Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook" Lubricants 8, no. 5: 50. https://doi.org/10.3390/lubricants8050050
APA StyleNifant’ev, I., Bagrov, V., Vinogradov, A., Vinogradov, A., Ilyin, S., Sevostyanova, N., Batashev, S., & Ivchenko, P. (2020). Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants, 8(5), 50. https://doi.org/10.3390/lubricants8050050