High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit
Abstract
:1. Introduction
2. Materials and Methods
2.1. MoS2 Solid Lubricant Coating
2.2. Friction Force Microscopy
2.3. Environmental Control for FFM
3. Results & Discussion
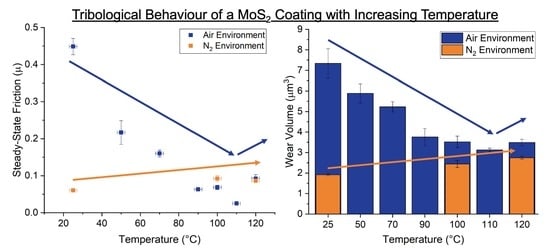
3.1. Friction Behaviour
3.2. Wear Mechanics
3.3. Run-in Characteristics
3.4. Implication for Mechanisms in LEO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, E.W. Space tribology: Its role in spacecraft mechanisms. J. Phys. D Appl. Phys. 2012, 45, 305001. [Google Scholar] [CrossRef]
- Fusaro, R.L. Lubrication of Space Systems. In Proceedings of the Society of Tribologists and Lubrication Engineers Annual Meeting, Pittsburgh, PA, USA, 1994; Available online: https://ntrs.nasa.gov/search.jsp?R=19940024896 (accessed on 21 April 2020).
- Fusaro, R.L.; Khonsari, M.M. Liquid Lubrication for Space Applications; NASA: Hanover, MD, USA, 1993. [Google Scholar]
- Antoniazzi, J.; Milligan, D. A Review of Lubrication on the Canadarm 2. In Protection of Materials and Structures from Space Environment; Springer: Berlin, Germany, 2004; pp. 291–298. [Google Scholar]
- Jones, W.; Jansen, M. Lubrication for Space Applications. Handb. Lubr. Tribol. 2010, 222, 997–1004. [Google Scholar]
- Godfrey, D.; Nelson, E. Oxidation Characteristics of Molybdenum Disulfide and Effect of Such Oxidation on its Role as a Solid-Film Lubricant; National Advisory Committee for Aeronautics, 1949. Available online: https://ntrs.nasa.gov/search.jsp?R=19930082560 (accessed on 21 April 2020).
- Savan, A.; Pflüger, E.; Voumard, P.; Schröer, A.; Paul, M.S. Modern solid lubrication: Recent developments and applications of MoS2. Lubr. Sci. 2000, 12, 185–203. [Google Scholar] [CrossRef]
- Spalvins, T. A review of recent advances in solid film lubrication. J. Vac. Sci. Technol. A 1987, 5, 212–219. [Google Scholar] [CrossRef]
- National Aeronautics and Space Administration. A Researcher’s Guide to the International Space Station; NASA: Hanover, MD, USA, 2004. [Google Scholar]
- Brizuela, M.; Oñate, J.I.; Garmendia, I. Tribolab: An Experiment on Space Tribology, In-Orbit Data at the ISS. In Proceedings of the Proc. ‘13th European Space Mechanisms and Tribology Symposium–ESMATS 2009, Vienna, Austria, 23–25 September 2009. [Google Scholar]
- Woods, T. Out of Thin Air. Available online: https://www.nasa.gov/topics/technology/features/atomic_oxygen.html. (accessed on 21 April 2020).
- Banks, B.A.; de Groh, K.K.; Miller, S.K. Low Earth Orbital Atomic Oxygen Interactions With Spacecraft Materials; NASA: Hanover, MD, USA, 2004. [Google Scholar]
- Gao, X.; Hu, M.; Sun, J.; Fu, Y.; Yang, J.; Liu, W.; Weng, L. Changes in the composition, structure and friction property of sputtered MoS 2 films by LEO environment exposure. Appl. Surf. Sci. 2015, 330, 30–38. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Matsumoto, K.; Suzuki, M.; Teraoka, Y.; Kitamura, A.; Belin, M.; Fontaine, J.; Martin, J.M. Space environmental effects on MoS2 and diamond-like carbon lubricating films: Atomic oxygen-induced erosion and its effect on tribological properties. Surf. Coat. Technol. 2007, 202, 1003–1010. [Google Scholar] [CrossRef]
- Argibay, N.; Dugger, M.T.; Krick, B.A.; Curry, J.F.; Nation, B.; Martini, A.; Strandwitz, N.C.; Babuska, T. Highly Oriented MoS2 Coatings: Tribology and Environmental Stability. Tribol. Lett. 2016, 64, 1–9. [Google Scholar]
- Wang, P.; Qiao, L.; Xu, J.; Li, W.; Liu, W. Erosion Mechanism of MoS2-Based Films Exposed to Atomic Oxygen Environments. ACS Appl. Mater. Interfaces 2015, 7, 12943–12950. [Google Scholar] [CrossRef]
- Tagawa, M.; Muromoto, M.; Hachiue, S.; Yokota, K.; Ohmae, N.; Matsumoto, K.; Suzuki, M. Hyperthermal atomic oxygen interaction with MoS2 lubricants and relevance to space environmental effects in low earth orbit—Effects on friction coefficient and wear-life. Tribol. Lett. 2005, 18, 437–443. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ochi, K.; Akiyama, M.; Matsumoto, K.; Suzuki, M. Comparison of macro and microtribological property of molybdenum disulfide film exposed to LEO space environment. Tribol. Lett. 2012, 45, 349–356. [Google Scholar] [CrossRef]
- Serpini, E.; Rota, A.; Ballestrazzi, A.; Marchetto, D.; Gualtieri, E.; Valeri, S. The role of humidity and oxygen on MoS2 thin films deposited by RF PVD magnetron sputtering. Surf. Coat. Technol. 2017, 319, 345–352. [Google Scholar] [CrossRef]
- Sliney, H.E. Solid lubricant materials for high temperatures—A review. Tribol. Int. 1982, 15, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.F.; Jiang, Y.; Hardell, J.; Prakash, B.; Fang, Q.F. Influence of service temperature on tribological characteristics of self-lubricant coatings: A review. Front. Mater. Sci. 2013, 7, 28–39. [Google Scholar] [CrossRef]
- Wright, M.C.; Long, V.L.; McDanels, S. The Evolution of Failure Analysis at NASA’s Kennedy Space Center and the Lessons Learned; NASA: Hanover, MD, USA, 2008. [Google Scholar]
- Gardos, M.N. Anomalous wear behavior of MoS2 films in moderate vacuum and dry nitrogen. Tribol. Lett. 1995, 1, 67–85. [Google Scholar] [CrossRef]
- Serles, P.; Sun, H.; Colas, G.; Tam, J.; Nicholson, E.; Wang, G.; Howe, J.; Saulot, A.; Singh, C.V.; Filleter, T. Structure Dependent Wear and Shear Mechanics of Nanostructured MoS2 Coatings. Adv. Mater. Interfaces 2020, in press. [Google Scholar]
- Sader, J.E.; Chon, J.W.M.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. [Google Scholar] [CrossRef] [Green Version]
- Green, C.P.; Lioe, H.; Cleveland, J.P.; Proksch, R.; Mulvaney, P.; Sader, J.E. Normal and torsional spring constants of atomic force microscope cantilevers. Rev. Sci. Instrum. 2004, 75, 1988–1996. [Google Scholar] [CrossRef] [Green Version]
- Cannara, R.J.; Eglin, M.; Carpick, R.W. Lateral force calibration in atomic force microscopy: A new lateral force calibration method and general guidelines for optimization. Rev. Sci. Instrum. 2006, 77, 053701. [Google Scholar] [CrossRef] [Green Version]
- Khare, H.S.; Burris, D.L. The effects of environmental water and oxygen on the temperature-dependent friction of sputtered molybdenum disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Kubart, T.; Polcar, T.; Kopecký, L.; Novák, R.; Nováková, D. Temperature dependence of tribological properties of MoS2 and MoSe2 coatings. Surf. Coat. Technol. 2005, 193, 230–233. [Google Scholar] [CrossRef]
- Arif, T.; Yadav, S.; Colas, G.; Singh, C.V.; Filleter, T. Understanding the Independent and Interdependent Role of Water and Oxidation on the Tribology of Ultrathin Molybdenum Disulfide (MoS2). Adv. Mater. Interfaces 2019, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Levita, G.; Restuccia, P.; Righi, M.C. Graphene and MoS2 interacting with water: A comparison by ab initio calculations. Carbon 2016, 107, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Savan, A.; Simmonds, M.C.; Huang, Y.; Constable, C.P.; Creasey, S.; Gerbig, Y.; Haefke, H.; Lewis, D.B. Effects of temperature on the chemistry and tribology of co-sputtered MoSx-Ti composite thin films. Thin Solid Films 2005, 489, 137–144. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. Surface and subsurface contributions of oxidation and moisture to room temperature friction of molybdenum disulfide. Tribol. Lett. 2014, 53, 329–336. [Google Scholar] [CrossRef]
- Curry, J.F.; Hinkle, A.R.; Babuska, T.F.; Wilson, M.A.; Dugger, M.T.; Krick, B.A.; Argibay, N.; Chandross, M. Atomistic Origins of Temperature-Dependent Shear Strength in 2D Materials. ACS Appl. Nano Mater. 2018, 1, 5401–5407. [Google Scholar] [CrossRef]
- Bandaru, N.; Kumar, R.S.; Sneed, D.; Tschauner, O.; Baker, J.; Antonio, D.; Luo, S.N.; Hartmann, T.; Zhao, Y.; Venkat, R. Effect of pressure and temperature on structural stability of MoS 2. J. Phys. Chem. C 2014, 118, 3230–3235. [Google Scholar] [CrossRef]
- Colbert, R.S.; Sawyer, G.W. Thermal dependence of the wear of molybdenum disulphide coatings. Wear 2010, 269, 719–723. [Google Scholar] [CrossRef]
- Babuska, T.F.; Pitenis, A.A.; Jones, M.R.; Nation, B.L.; Sawyer, W.G.; Argibay, N. Temperature-Dependent Friction and Wear Behavior of PTFE and MoS2. Tribol. Lett. 2016, 63, 1–7. [Google Scholar] [CrossRef]
- Kazachenko, V.P.; Popov, V.V.; Dubravin, A.M.; Ahn, H.-S.; Chizhik, S.A. Application of phase contrast imaging atomic force microscopy to tribofilms on DLC coatings. Wear 2002, 249, 617–625. [Google Scholar]
- Ye, J.; Kano, M.; Yasuda, Y. Determination of nanostructures and mechanical properties on the surface of molybdenum dithiocarbamate and zinc dialkyl-dithiophosphate tribochemical reacted films using atomic force microscope phase imaging technique. J. Appl. Phys. 2003, 93, 5113–5117. [Google Scholar] [CrossRef]
- Blau, P.J. On the nature of running-in. Tribol. Int. 2005, 38, 1007–1012. [Google Scholar] [CrossRef]
- Fleischauer, P.D. Effects of crystallite orientation on environmental stability and lubrication properties of sputtered moS. ASLE Trans. 1984, 27, 82–88. [Google Scholar] [CrossRef]
- Moser, J.; Levy, F. Growth Mechanisms and Near-Interface Structure in Relation To Orientation of Mos2 Sputtered Thin-Films. J. Mater. Res. 1992, 7, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Furlan, K.P.; de Mello, J.D.B.; Klein, A.N. Self-lubricating composites containing MoS2: A review. Tribol. Int. 2018, 120, 280–298. [Google Scholar] [CrossRef]
- Cowen, R. The wheels come off Kepler. Nature 2013, 497, 417–418. [Google Scholar] [CrossRef] [Green Version]
- Aldridge, D.; Gentilhomme, M.; Gibson, A.; Cameron, P.; Mccolgan, A. Cryogenic Motor Enhancement for the Niriss Instrument on the James Webb Space Telescope. In Proceedings of the 16th European Space Mechanisms and Tribology Symposium, Bilbao, Spain, 23–25 September 2015. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serles, P.; Gaber, K.; Pajovic, S.; Colas, G.; Filleter, T. High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit. Lubricants 2020, 8, 49. https://doi.org/10.3390/lubricants8040049
Serles P, Gaber K, Pajovic S, Colas G, Filleter T. High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit. Lubricants. 2020; 8(4):49. https://doi.org/10.3390/lubricants8040049
Chicago/Turabian StyleSerles, Peter, Khaled Gaber, Simo Pajovic, Guillaume Colas, and Tobin Filleter. 2020. "High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit" Lubricants 8, no. 4: 49. https://doi.org/10.3390/lubricants8040049
APA StyleSerles, P., Gaber, K., Pajovic, S., Colas, G., & Filleter, T. (2020). High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit. Lubricants, 8(4), 49. https://doi.org/10.3390/lubricants8040049