Effective Application of Solid Lubricants in Spacecraft Mechanisms
Abstract
:| Content | |
| 1. Introduction | 3 |
| 2. Material Properties, Structure, and Lubrication Mechanisms | 4 |
| 2.1. Transition Metal Dichalcogenides—MoS2 and WS2 | 5 |
| 2.2. PTFE | 6 |
| 2.3. Surface Reaction Layers | 7 |
| 3. Application and Use of Solid Lubricant Formulations | 7 |
| 3.1. Surface Pretreatment for Thin Lubricant Coatings | 9 |
| 3.2. Unbonded/Burnished Coatings | 9 |
| 3.2.1. Special Case: Air-impinged MoS2 and WS2 | 10 |
| 3.3. Bonded Coatings | 11 |
| 3.3.1. Heat-cured resin-bonded coatings | 13 |
| 3.3.2. Air-cured resin-bonded coatings | 14 |
| 3.3.3. Inorganic-bonded Coatings (nonceramic) | 14 |
| 3.3.4. Ceramic-bonded Coatings | 14 |
| 3.4. Sputter-Deposited Coatings | 15 |
| 3.5. Metal Coatings | 19 |
| 3.6. PVD/CVD-Deposited Hard Coatings | 20 |
| 3.7. Ion-Implantation | 21 |
| 3.8. Composite Materials | 22 |
| 4. Optimized Solid-Lubricated Contact Design | 25 |
| 5. Testing of Lubricated Devices | 26 |
| 5.1. Standard Tests | 27 |
| 5.2. Testing Strategies for Flight Parts | 27 |
| 5.3. Qualification by Similarity/Requirement Creep | 28 |
| 6. Potential Challenges to Successful Application of Tribological Solids | 28 |
| 6.1. Humid Air Sensitivity of Solid Lubricants during Storage | 28 |
| 6.1.1. Measurement of Oxidation on MoS2 Powders after Long-Term Humid Air Exposure | 29 |
| 6.1.2. Tribological Degradation of Sputter-Deposited Nanocomposite MoS2 Coatings after Long Term Humid Air Exposure | 30 |
| 6.1.3. Effect of moisture absorption on inorganic-bonded coatings | 32 |
| 6.2. Humid Air Sensitivity of MoS2 during Operation | 33 |
| 6.3. LOX Compatibility | 34 |
| 6.4. Graphite | 34 |
| 6.5. Thermal effects | 34 |
| 6.6. Atomic oxygen exposure | 36 |
| 6.7. Materials compatibility | 36 |
| 6.8. Tolerance budgets | 37 |
| 6.9. Wear debris | 37 |
| 7. Typical Applications for Solid Lubricants/Antiwear Coatings in Spacecraft | 37 |
| 7.1. Actuators | 37 |
| 7.2. Deployment and Release Mechanisms | 38 |
| 7.3. Solid-Lubricated Slip Ring Assemblies | 39 |
| 7.3.1. Fabrication and Testing of Solid-Lubricated Slip Ring Assemblies | 40 |
| 7.3.2. Atmospheric Degradation during Storage/Testing | 41 |
| 7.4. Other applications | 42 |
| 8. Future of Tribological Solids on Spacecraft | 43 |
| 8.1. Highly Hydrogenated Diamond-Like Carbon (HH-DLC) | 43 |
| 8.2. Cubic boron nitride (c-BN) | 44 |
| 8.3. Surface Microtexturing | 44 |
| 8.4. Adaptive “Chameleon” Lubrication | 45 |
| 8.5. Hybrid Liquid/Solid Lubrication | 46 |
| 9. Conclusions | 46 |
| References | 47 |
1. Introduction
- Low to medium numbers of duty cycles
- Moderately-high to low contact stresses
- Extreme environments
- Low speed boundary contacts
2. Material Properties, Structure, and Lubrication Mechanisms
2.1. Transition Metal Dichalcogenides—MoS2 and WS2
2.2. PTFE
2.3. Surface Reaction Layers
3. Application and Use of Solid Lubricant Formulations
3.1. Surface Pretreatment for Thin Lubricant Coatings
3.2. Unbonded/Burnished Coatings
3.2.1. Special Case: Air-impinged MoS2 and WS2
3.3. Bonded Coatings
- Timken T54148 test rings were coated with phenolic resin-bonded MoS2/graphite coatings [45]. Samples were tested on the LFW-1 test apparatus (block sliding on ring) at 72 rpm (0.87 mm/s) and 630 lb load. With no pretreatment, the coating failed on loading. Two other samples underwent vapor degreasing followed by sandblasting, but the second one was subsequently treated to an additional phosphate treatment before coating. The first failed at 2 × 104 cycles, while the phosphate-treated surface lasted to 6.7 × 105 cycles.
- Journal bearing tests were conducted using phenolic resin-bonded MoS2 coatings [49]. Contact pressures were 3–4 ksi, with 0.87 mm/s sliding speed. In that study, coatings deposited on 304 CRES exhibited wear lives 3–10 times greater than those on 440 C CRES. Both steels underwent the same pretreatment (grit-blasting followed by passivation). However, the 304 CRES surface was rougher, so that the adhesion of the lubricant coating was greater.
3.3.1. Heat-Cured Resin-Bonded Coatings
3.3.2. Air-Cured Resin-Bonded Coatings
3.3.3. Inorganic-Bonded Coatings (Nonceramic)
3.3.4. Ceramic-Bonded Coatings
3.4. Sputter-Deposited Coatings
3.5. Metal Coatings
3.6. PVD/CVD-Deposited Hard Coatings
3.7. Ion-Implantation
3.8. Composite Materials
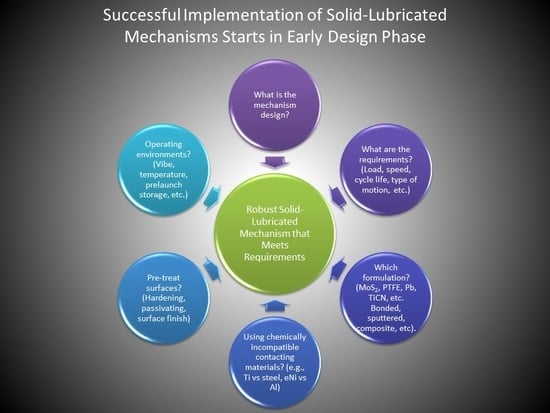
4. Optimized Solid-Lubricated Contact Design
- Materials and chemical properties of the device surfaces
- Hertzian contact stress (Smax)
- Type and duration of relative contact motion
- Environment (gaseous/vacuum, temperature, launch)
5. Testing of Lubricated Devices
5.1. Standard Tests
5.2. Testing Strategies for Flight Parts
5.3. Qualification by Similarity/Requirement Creep
6. Potential Challenges to Successful Application of Tribological Solids
6.1. Humid Air Sensitivity of Solid Lubricants during Storage
6.1.1. Measurement of Oxidation on MoS2 Powders after Long-Term Humid Air Exposure
6.1.2. Tribological Degradation of Sputter-Deposited Nanocomposite MoS2 Coatings after Long Term Humid Air Exposure
6.1.3. Effect of Moisture Absorption on Inorganic-Bonded Coatings
6.2. Humid Air Sensitivity of MoS2 during Operation
6.3. LOX Compatibility
6.4. Graphite
6.5. Thermal Effects
6.6. Atomic Oxygen Exposure
6.7. Materials Compatibility
6.8. Tolerance Budgets
6.9. Wear Debris
7. Typical Applications for Solid Lubricants/Antiwear Coatings in Spacecraft
7.1. Actuators
7.2. Deployment and Release Mechanisms
7.3. Solid-Lubricated Slip Ring Assemblies
7.3.1. Fabrication and Testing of Solid-Lubricated Slip Ring Assemblies
7.3.2. Atmospheric Degradation during Storage/Testing
7.4. Other Applications
8. Future of Tribological Solids on Spacecraft
8.1. Highly Hydrogenated Diamond-Like Carbon (HH-DLC)
8.2. Cubic Boron Nitride (c-BN)
8.3. Surface Microtexturing
8.4. Adaptive “Chameleon” Lubrication
8.5. Hybrid Liquid/Solid Lubrication
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, M.E. Solid Lubricants: A Survey; NASA SP-5059(01); NASA: Washington, DC, USA, 1972. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19720017826.pdf (accessed on 15 February 2020).
- Lince, J.R.; Fleischauer, P.D. Solid Lubricants (Chap. 7). In Space Vehicle Mechanisms: Elements of Successful Design; Conley, P., Ed.; Wiley-Interscience: New York, NY, USA, 1998. [Google Scholar]
- Luciano, G. Cryogenic Mechanism of ISO Camera. In Proceedings of the 3th European Space Mechanisms and Tribology Symposium., Madrid, Spain, 30 September–2 October 1987; p. 233. [Google Scholar]
- Poole, W.E.; Bursey, R.W., Jr. Pratt & Whitney Cryogenic Turbopump Bearing Experience. In Advanced Earth-to-Orbit Propulsion Technology; NTIS No. N90-28624; NASA, Marshall Space Flight Center: Huntsville, AL, USA, 1988. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900019308.pdf (accessed on 15 February 2020).
- Miyoshi, K. Solid Lubricants and Coatings for Extreme Environments: State-of-the-Art Survey; NASA/TM—2007-214668; NASA: Cleveland, OH, USA, 2007. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20070010580.pdf (accessed on 15 February 2020).
- Lince, J.R. Electrical Contact Ring Assemblies. Chap. 16. In NASA Space Mechanisms Handbook; NASA/TP-1999-206988; Fusaro, R.L., Ed.; Glenn Research Center: Cleveland, OH, USA, 1999. [Google Scholar]
- Roberts, E.W. A Review of Sliding Electrical Contacts for Space Application; ESA Contract Report No. ESA(ESTL)52; ESA: Paris, France, 1981.
- Menzel, K.; Jung, H.J.; Schmidt, J. Development of an Actuator for Ambient to Cryo Application. In Proceedings of the 40th Aerospace Mechanisms Symposium, Cocoa Beach, FL, USA, 12–14 May 2010; pp. 389–400. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20100021922.pdf (accessed on 15 February 2020).
- Loewenthal, S.H.; Chou, R.G.; Hopple, G.B.; Wenger, W.L. Evaluation of Ion-Sputtered Molybdenum Disulfide Bearings for Spacecraft Gimbals. Trib. Trans. 1994, 37, 505–515. [Google Scholar] [CrossRef]
- Haslehurst, A.; Lewis, S. The Use of Dry Lubricated Bearings in Reaction Wheels. In Proceedings of the 14th European Space Mechanisms & Tribology Symposium—ESMATS 2011, Constance, Germany, 28–30 September 2011; pp. 253–261. Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2011/haslehurst.pdf (accessed on 15 February 2020).
- Roberts, E.W. Space Tribology: Its Role in Spacecraft Mechanisms. J. Phys. D Appl. Phys. 2012, 45, 503001. [Google Scholar] [CrossRef]
- Lince, J.R.; Loewenthal, S.H.; Clark, C.S. Tribological and Chemical Effects of Long Term Humid Air Exposure on Sputter-Deposited Nanocomposite MoS2 Coatings. Wear 2019, 432–433, 202935. [Google Scholar] [CrossRef]
- Lince, J.R.; Loewenthal, S.H.; Clark, C.S. Degradation of Sputter-Deposited Nanocomposite MoS2 Coatings for NIRCam during Storage in Air. In Proceedings of the 43rd Aerospace Mechanisms Symposium, Santa Clara, CA, USA, 4–6 May 2016; Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2016/lince.pdf (accessed on 15 February 2020).
- Krantz, T.; Hakun, C.; Cameron, Z.; Shareef, I.; Dube, M. Performance of MoS2 Coated Gears Exposed to Humid Air During Storage. In Proceedings of the 44th Aerospace Mechanisms Symposium, Cleveland, OH, USA, 16–18 May 2018; Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2018/krantz.pdf (accessed on 15 February 2020).
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid Lubrication with MoS2: A Review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Sherbiney, M.A.; Halling, J. Friction and Wear of Ion-Plated Soft Metallic Films. Wear 1977, 45, 211. [Google Scholar] [CrossRef]
- Villa, D.; Toledo, G. Launch Lock Mechanism Design Fault Tree Use and Coatings Study. In Proceedings of the 39th Aerospace Mechanisms Symposium, Huntsville, AL, USA, 7–9 May 2008; Available online: http://www.esmats.eu/amspapers/pastpapers/pdfs/2008/villa.pdf (accessed on 15 February 2020).
- Hilton, M.R.; Fleischauer, P.D. Lubricants for High-Vacuum Applications. In ASM Handbook, Volume 18: Friction, Lubrication, and Wear Technology; Henry, D., Ed.; ASM International: Materials Park, OH, USA, 1992; p. 150. [Google Scholar]
- McMurtrey, E.L. High Performance Solid and Liquid Lubricants; Noyes Data Corp.: Park Ridge, NJ, USA, 1987. [Google Scholar]
- Sliney, H.E. Solid Lubricants. In ASM Handbook, Volume 18: Friction, Lubrication and Wear Technology; Henry, D., Ed.; ASM International: Materials Park, OH, USA, 1992; p. 113. [Google Scholar]
- Shackelford, J.F. (Ed.) CRC Materials Science and Engineering Handbook; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Le Mogne, T.; Donnet, C.; Martin, J.M.; Tonck, A.; Millard-Pinard, N.; Fayeulle, S.; Moncoffre, N. Nature of Super-Lubricating MoS2 Physical Vapor Deposition Coatings. J. Vac. Sci. Technol. A 1994, 12, 1998. [Google Scholar] [CrossRef]
- Martin, J.M.; Donnet, C.; Le Mogne, T.; Epicier, T. Superlubricity of Molybdenum Disulphide. Phys. Rev. B 1993, 48, 10583. [Google Scholar] [CrossRef]
- Baykara, M.Z.; Vazirisereshk, M.R.; Martini, A. Emerging superlubricity: A review of the state of the art and perspectives on future research. Appl. Phys. Rev. 2018, 5, 041102. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, J.H.; Gao, S.; Chen, Q.; Peng, L.M.; Liu, K.H.; Wei, X.L. Superlubricity between MoS2 Monolayers. Adv. Mater. 2017, 29, 1701474. [Google Scholar] [CrossRef]
- Singer, I.L.; Bolster, R.N.; Wegand, J.; Fayeulle, S.; Stupp, B.C. Hertzian stress contribution to low friction behavior of thin MoS2 coatings. Appl. Phys. Lett. 1990, 57, 995–997. [Google Scholar] [CrossRef]
- Amontons, G. Memoires de l’Academie Royale des Sciences; Chez Gerard Kyuper: Amsterdam, The Netherlands, 1706; pp. 257–282. [Google Scholar]
- Roberts, E.W. Institute of Mechanical Engineering Tribology—Friction, Lubrication and Wear, Fifty Years On; Institute of Mechanical Engineering: London, UK, 1987; p. 503. [Google Scholar]
- Grosseau-Poussard, J.L.; Moine, P.; Brendle, M. Shear strength measurements of parallel MoSx thin films. Thin Solid Films 1997, 307, 163–168. [Google Scholar] [CrossRef]
- Lince, J.R. Tribology of Co-Sputtered Nanocomposite Au/MoS2 Solid Lubricant Films over a Wide Contact Stress Range. Trib. Lett. 2004, 17, 419–428. [Google Scholar] [CrossRef]
- Anderson, M.J.; Cropper, M.; Roberts, E.W. The Tribological Characteristics of Dicronite. In Proceedings of the European Space Mechanisms and Tribology Symposium (ESMATS), Liverpool, UK, 19–21 September 2007; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2007/anderson.pdf (accessed on 15 February 2020).
- Jansson, M.; Koenen, J.; Viviente, J.-L.; Tvaruzka, A.; Merstallinger, A. Development of Dry Lubricated Harmonic Drives for Space Applications (“harmLES”). In Proceedings of the 15th European Space Mechanisms and Tribology Symposium (ESMATS 2013), Noordwijk, The Netherlands, 25–27 September 2013; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2013/jansson.pdf (accessed on 15 February 2020).
- Briscoe, B.J.; Tabor, D. Friction and Wear of Polymers. In Polymer Surfaces; Clark, D.T., Frost, W.J., Eds.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Dowson, D. Tribological Characteristics of Polymers with Particular Reference to Polyethylene. In Polymer Surfaces; Clark, D.T., Frost, W.J., Eds.; John Wiley & Sons: New York, NY, USA, 1978; p. 399. [Google Scholar]
- Briscoe, B.J.; Pooley, C.M.; Tabor, D. Friction and Transfer of Some Polymers in Unlubricated Sliding. In Advances in Polymer Friction and Wear; Lee, L.-H., Ed.; Plenum Press: New York, NY, USA, 1974; p. 191. [Google Scholar]
- Schönherr, H.; Vancso, G.J. The mechanism of PTFE and PE friction deposition: A combined scanning electron and scanning force microscopy study on highly oriented polymeric sliders. Polymer 1998, 39, 5705–5709. [Google Scholar] [CrossRef]
- Hard Anodic Coating on Aluminum and Aluminum Alloys; AMS2469J; SAE International: Warrendale, PA, USA, 2014; Available online: https://www.sae.org/standards/content/ams2469j/ (accessed on 15 February 2020).
- Anodic Treatment—Titanium and Titanium Alloys, Solution pH 13 or Higher; AMS2488E; SAE International: Warrendale, PA, USA, 2019; Available online: https://www.sae.org/standards/content/ams2488e/ (accessed on 15 February 2020).
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Pilkey, W.D. Formulas for Stress, Strain, and Structural Matrices, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Available online: https://www.amesweb.info/HertzianContact/HertzianContact.aspx;https://www.mesys.ch/calc/hertz.fcgi?lang=en (accessed on 15 February 2020).
- Kannel, J.W.; Lowry, J.A.; Dufrane, K.F. Lubricant Selection Manual, Phase III; NASA-CR-184363; NASA: Battelle, Columbus, OH, USA, 1991. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19920024392.pdf (accessed on 15 February 2020).
- Lubricant Application, Solid Film, Heat Cured, Corrosion Inhibiting, Procurement Specification, AS5528D; SAE International: Warrendale, PA, USA, 2020; Available online: https://www.sae.org/standards/content/as5528d/ (accessed on 15 February 2020).
- Clauss, F.J. Solid Lubricants and Self-Lubricating Solids; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Johnston, R.R.M.; Moore, A.J.W. The Burnishing of Molybdenum Disulphide on to Metal Surfaces. Wear 1964, 19, 498–512. [Google Scholar] [CrossRef]
- Gresham, R.M. Bonded Solid Film Lubricants. CRC Handbook of Lubrication and Tribology, Volume III, Monitoring, Materials, Synthetic Lubricants, and Applications; Booser, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Lancaster, J.K. Solid Lubricants. In CRC Handbook of Lubrication (Theory and Practice of Tribology), Volume II: Theory and Design; Richard Booser, F., Ed.; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Lubricant, Solid Film, Heat Cured, Corrosion Inhibiting; MIL-L-46010E; Defense Quality and Standardization Office: Falls Church, VA, USA, 1997.
- Lubricant, Solid Film, Air Cured, Corrosion Inhibiting; MIL-L-46147A; Defense Quality and Standardization Office: Falls Church, VA, USA, 1990.
- Haraoka, N.; Sasaki, A.; Kawashima, N.; Honda, T. Wear Characteristics of Bonded Solid Film Lubricant under High Load Condition. In Proceedings of the 25th AEROSPACE MECHANISMS SYMPOSIUM, Pasadena, CA, USA, 8–10 May 1991; p. 179. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19910015302.pdf (accessed on 15 February 2020).
- Lubricant, Solid Film; AS1701F; SAE International: Warrendale, PA, USA, 2017; Available online: https://www.sae.org/standards/content/as1701f/ (accessed on 15 February 2020).
- Hopkins, V.; Campbell, M. Film Thickness Effect on the Wear Life of a Bonded Solid Lubricant Film. ASLE Trans. 1969, 25, 15. [Google Scholar]
- Gresham, R.M. Bonded Solid Film Lubricants for Fastener Coatings; Fastener Technol. Int.: Akron, OH, USA, 1987. [Google Scholar]
- Centers, P.W. The Role of Oxide and Sulfide Additions in Solid Lubricant Compacts. Tribol. Trans. 1988, 31, 149. [Google Scholar] [CrossRef]
- Gänsheimer, J. Bonded Solid Lubricants: Features, Benefits, Limitations. Oxid. Commun. 1995, 18, 82. [Google Scholar]
- Zhao, H.; Chang, S.K. Dry Lubricant Performance on Spur Gears for High-Cycle Space Applications. Spar J. Eng. Technol. 1994, 3, 68. [Google Scholar]
- Allmon, C.; Haugen, B. Space Station Freedom Solar Array Tension Mechanism Development. In Proceedings of the 28th Aerospace Mechanisms Symposia, Cleveland, OH, USA, 18–20 May 1994; p. 123. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028794.pdf (accessed on 15 February 2020).
- Standard Test Method for Total Mass Loss and Collected Volatile Condensable Materials from Outgassing in a Vacuum Environment; ASTM E595-15; ASTM Int.: West Conshohocken, PA, USA, 2015.
- Riggs, B. Mars Science Laboratory Rover Mobility Bushing Development. In Proceedings of the 39th Aerospace Mechanisms Symposium, Huntsville, AL, USA, 7–9 May 2008; pp. 83–96. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2008/riggs.pdf (accessed on 15 February 2020).
- Sliney, H.L. Status and New Directions for Solid Lubricant Coatings and Composite Materials. In Tribology in the 80′s, Volume 2; NASA Lewis Research Center: ProcCleveland, OH, USA, 1983; pp. 665–680. [Google Scholar]
- Sliney, H.E. Wide Temperature Spectrum Self-Lubricating Coatings Prepared by Plasma Spraying. Thin Solid Films 1979, 64, 211–217. [Google Scholar] [CrossRef] [Green Version]
- DellaCorte, C.; Edmonds, B.J. NASA PS400: A New High Temperature Solid Lubricant Coating for High Temperature Wear Applications; NASA Technical Memorandum NASA/TM—2009-215678; Glenn Research Center: Cleveland, OH, USA, 2009. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20090033769.pdf (accessed on 15 February 2020).
- Spalvins, T. Lubrication with Sputtered MoS2 Films. ASLE Trans. 1971, 14, 267. [Google Scholar] [CrossRef] [Green Version]
- Renevier, N.M.; Fox, V.C.; Teer, D.G.; Hampshire, J. Coating characteristics and tribological properties of sputter-deposited MoS2/metal composite coatings deposited by closed field unbalanced magnetron sputter ion plating. Surf. Coat. Technol. 2000, 127, 24–37. [Google Scholar] [CrossRef]
- Hilton, M.R.; Bauer, R.; Didziulis, S.V.; Lince, J.R.; Fleischauer, P.D. Structural, Chemical, and Tribological Studies of Sputter-Deposited MoS2 Solid Lubricant Films. In Advances in Engineering Tribology; ASLE SP-31; Chung, Y.W., Cheng, H.S., Eds.; STLE: Park Ridge, IL, USA, 1991; p. 31. [Google Scholar]
- Hilton, M.R.; Fleischauer, P.D. Structural Studies of Sputter-Deposited MoS2 Solid Lubricant Films. In New Materials Approaches to Tribology: Theory and Applications. Mater. Res. Soc. Symp. Proc. 1989, 140, 227. [Google Scholar] [CrossRef]
- Roberts, E.W.; Williams, B.J.; Ogilvy, J.A. The Effect of Surface Roughness on the Friction and Wear Properties of Sputtered MoS2 Films. J. Phys. D Appl. Phys. 1992, 25, A65. [Google Scholar] [CrossRef]
- Lince, J.R.; Fleischauer, P.D. Crystallinity of Rf-Sputtered MoS2 Films. J. Mater. Res. 1987, 2, 827. [Google Scholar] [CrossRef]
- Lince, J.R.; Hilton, M.R.; Bommannavar, A.S. Oxygen Substitution in Sputter-Deposited MoS2 Films studied by Extended X-Ray Absorption Fine Structure, X-Ray Photoelectron Spectroscopy, and X-Ray Diffraction. Surf. Coat. Technol. 1990, 43–44, 640. [Google Scholar] [CrossRef]
- Lince, J.R.; Hilton, M.R.; Bommannavar, A.S. EXAFS of Sputter-Deposited MoS2 Films. Thin Solid Films 1995, 264, 120. [Google Scholar] [CrossRef]
- Buck, V. Lattice Parameters of Sputtered MoS2 Films. Thin Solid Films 1991, 198, 157. [Google Scholar] [CrossRef]
- Dimigen, H.; Hubsch, H.; Willich, P.; Reichelt, K. Stoichiometry and Friction Properties of Sputtered MoSx Layers. Thin Solid Films 1985, 129, 79. [Google Scholar] [CrossRef]
- Lince, J.R. Doped MoS2 Coatings and Their Tribology. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Deepthi, B.; Barshilia, H.C. Nanostructured Solid Lubricant Coatings for Aerospace Applications. In Aerospace Materials Handbook, 1st ed.; Zhang, S., Zhao, D., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Spalvins, T. Frictional and Morphological Properties of Au-MoS2 Films Sputtered from a Compact Target. Thin Solid Films 1984, 118, 375. [Google Scholar] [CrossRef] [Green Version]
- Stupp, B.C. Performance of Conventionally Sputtered MoS2 Versus Cosputtered MoS2 and Nickel; ASLE SP-14; STLE: Park Ridge, IL, USA, 1984; p. 217. [Google Scholar]
- Hilton, M.R.; Bauer, R.; Didziulis, S.V.; Dugger, M.T.; Keem, J.; Scholhamer, J. Structural and Tribological Studies of MoS2 Solid Lubricant Films Having Metal-Multilayer Nanostructures. Surf. Coat. Technol. 1992, 53, 13. [Google Scholar] [CrossRef]
- Niederhauser, P.; Hintermann, H.E.; Maillat, M. Moisture Resistant MoS2 based Composite Lubricant Films. Thin Solid Films 1983, 108, 209. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Donley, M.S.; Walck, S.D.; Schneider, T.R.; McDevitt, N.T. The effects of dopants on the chemistry and tribology of sputter-deposited MoS2 films. Tribol. Trans. 1995, 38, 894–904. [Google Scholar] [CrossRef]
- Hilton, M.R.; Jayaram, G.; Marks, L.D. Microstructure of Sputter-Deposited Metal- and Oxide-MoS2 Solid Lubricant Thin Films. J. Mater. Res. 1998, 13, 1022. [Google Scholar] [CrossRef]
- Lince, J.R.; Hilton, M.R.; Bommannavar, A.S. Metal Incorporation in Sputter-Deposited MoS2 Films studied by Extended X-ray Absorption Fine Structure. J. Mater. Res. 1995, 10, 2091. [Google Scholar] [CrossRef]
- Scharf, T.W.; Kotula, P.G.; Prasad, S.V. Friction and wear mechanisms in MoS2/Sb2O3/Au nanocomposite coatings. Acta Mater. 2010, 58, 4100–4109. [Google Scholar] [CrossRef]
- Kim, H.I.; Lince, J.R. Direct visualization of sliding-induced tribofilm on Au/MoS2 nanocomposite coatings by c-AFM. Tribol. Lett. 2007, 26, 61–65. [Google Scholar] [CrossRef]
- Wahl, K.J.; Dunn, D.N.; Singer, I.L. Wear behavior of Pb–Mo–S solid lubricating coatings. Wear 1999, 230, 175–183. [Google Scholar] [CrossRef]
- Nouadji, M.; Attaf, A.; El Abdi, R.; Poulain, M. Study of glass formation in the Sb2O3–PbO–MnO ternary system. J. Alloys Compd. 2012, 511, 209–214. [Google Scholar] [CrossRef]
- Roberts, E.W. Thin Solid-Lubricant Films in Space. In Flight-Vehicle Materials, Structures, and Dynamics, Volume 4; Fusaro, R.L., Achenbach, J.D., Eds.; ASME: New York, NY, USA, 1992. [Google Scholar]
- Roberts, E.W.; Watters, R.B.; Gill, S.; Birner, R.; Lange, G.; Posselt, W. Development of Long-Life, Low-Noise Linear Bearings for Atmospheric Interferometry. In Proceedings of the 28th Aerospace Mechanisms Symposia, Cleveland, OH, USA, 18–20 May 1994; p. 245. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028806.pdf (accessed on 15 February 2020).
- Johnson, M.E.; Haugen, B.; Anderson, G. Space Station Freedom Solar Array Containment Box Mechanisms. In Proceedings of the 28th Aerospace Mechanisms Symposia, Cleveland, OH, USA, 18–20 May 1994; p. 1. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028786.pdf (accessed on 15 February 2020).
- Okon, A.B. Mars Science Laboratory Drill. In Proceedings of the 40th Aerospace Mechanisms Symposium, Cocoa Beach, FL, USA, 12–14 May 2010; pp. 1–16. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2010/okon.pdf (accessed on 15 February 2020).
- Thiel, M.; Stöcker, J.; Rohe, C.; Kömle, N.I.; Kargl, G.; Hillenmaier, O.; Lell, P. The Rosetta Lander Anchoring System. In Proceedings of the 10th European Space Mechanisms & Tribology Symposium—ESMATS 2003, San Sebastian, Spain, 24–26 September 2003; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2003/thiel.pdf (accessed on 15 February 2020).
- Heinrich, B.; Zemann, J.; Rottmeier, F. Development of the Bepi Columbo MPO Solar Array Drive Assembly (SADA). In Proceedings of the 14th European Space Mechanisms & Tribology Symposium—ESMATS 2011, Constance, Germany, 28–30 September 2011; pp. 201–207. Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2011/heinrich.pdf (accessed on 15 February 2020).
- Roberts, E.W.; Anderson, M.J. Advances in Solid Lubricant Coating Technology. In Proceedings of the 9th European Space Mechanisms & Tribology Symposium—ESMATS 2001, Liege, Belgium, 19–21 September 2001; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2001/robertsanderson.pdf (accessed on 15 February 2020).
- Roberts, E.W. Thin Solid Lubricant Films in Space. Tribol. Int. 1990, 23, 95. [Google Scholar] [CrossRef]
- Todd, M.J.; Bentall, R.H. Lead Film Lubrication in Vacuum. In Proceedings of the 2nd International Conference on Solid Lubrication, ASLE SP-6, Denver, Colorado, 7–10 August 1984; p. 1984. [Google Scholar]
- Buttery, M. An Evaluation of Liquid, Solid, and Grease Lubricants for Space Mechanisms Using a Spiral Orbit Tribometer. In Proceedings of the 40th Aerospace Mechanisms Symposium, Cocoa Beach, FL, USA, 12–14 May 2010; pp. 59–72. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2010/buttery.pdf (accessed on 15 February 2020).
- Batista, J.; Vise, J.; Young, K. Roll Ring Assemblies for the Space Station. In Proceedings of the 28th Aerospace Mechanisms Symposium, Cleveland, OH, USA, 18–20 May 1994; p. 35. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028788.pdf (accessed on 15 February 2020).
- Ajayi, O.O.; Erdemir, A.; Hsieh, J.-H.; Erck, R.A.; Fenske, G.R.; Nichols, F.A. Boundary Film for Structural Ceramic Materials. Wear 1993, 162–164, 1150. [Google Scholar] [CrossRef] [Green Version]
- Space Tribology Handbook; Sections 6.1.3 & 6.2.2; Roberts, E. (Ed.) European Space Tribology Laboratory (ESTL): Warrington, Cheshire, UK, 1997. [Google Scholar]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications; Noyes: Westwood, NJ, USA, 1996. [Google Scholar]
- Boving, H.J.; Haenni, W.; Hintermann, H.-E. Titanium Carbide Coatings for Aerospace Ball Bearings. In Proceedings of the 22nd Aerospace Mechanisms Symposium, NASA Langley Research Center, Hampton, VA, USA, 4–6 May 1988; pp. 245–252. Available online: https://ntrs.nasa.gov/search.jsp?R=19880012102 (accessed on 15 February 2020).
- TiC Coated Bearing Balls. Available online: https://www.brycoat.com/surface-engineering/cvd-coatings/tic-coated-bearing-balls/ (accessed on 6 June 2020).
- Semiatin, S.L. ASM Handbook, Volume 14B—Metalworking: Sheet Forming—17.6.4 Carbon Steel Performance for Tooling Component; ASM International: Materials Park, OH, USA, 2006. [Google Scholar]
- Rai, A.K.; Massey, M.L.; Gschwender, L.J.; Snyder, C.E., Jr.; Zabinski, J.S.; Sharma, S.K.; Jones, W.R., Jr. Enhanced Performance of Pennzane® Greases for Space Applications by Both Additive Formulations and Smooth Hard Coatings. Trib. Trans. 2001, 44, 678–684. [Google Scholar] [CrossRef]
- Fontaine, J.; Le Mogne, T.; Vargiolu, R.; Bodovillé, G. Ultra-High Vacuum Friction Experiments to Simulate Material Transfer and Wear of the Platinum-Rhodium Proof Mass used in the Gradio Accelerometer. In Proceedings of the 10th European Space Mechanisms & Tribology Symposium—ESMATS 2017, San Sebastian, Spain, 24–26 September 2003; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2003/fontaine.pdf (accessed on 15 February 2020).
- Gangopadhyay, A. Friction and Wear of Hard Thin Coatings. In Tribology Data Handbook; Booser, E.R., Ed.; STLE: Park Ridge, IL, USA, 1997; Chapter 56. [Google Scholar]
- Ahmed, R. Rolling Contact Fatigue. In ASM Handbook, Volume 11: Failure Analysis and Prevention, Chapter on Wear Failures; Becker, W.T., Shipley, R.J., Eds.; ASM International: Materials Park, OH, USA, 2002; p. 113. Available online: https://doi-org_proxy.dotlib.com.br/10.31399/asm.hb.v11.9781627081801 (accessed on 15 February 2020).
- Valori, R.; Popgoshev, D. Ion implanting bearing surfaces for corrosion resistance. In Proceedings of the ASME/ASLE Lubrication Conference, Washington, DC, USA, 5–7 October 1982. [Google Scholar]
- Samandi, M.; Shedden, B.A.; Bell, T.; Collins, G.A.; Hutchings, R.; Tendys, J. Significance of Nitrogen Mass Transfer Mechanism on the Nitriding Behavior of Austenitic Stainless Steel. J. Vac. Sci. Technol. B 1994, 12, 935. [Google Scholar] [CrossRef]
- Hirvonen, J.K. Ion Implantation in Tribology and Corrosion Science. J. Vac. Sci. Technol. 1978, 15, 1662. [Google Scholar] [CrossRef]
- Gardos, M.N. Self-Lubricating Composites for Extreme Lubricating Conditions. In Friction and Wear of Polymer Composites, Volume 1; Friedrich, K., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1986; p. 397. [Google Scholar]
- Rohatgi, P.K.; Ray, S.; Liu, Y. Metal Matrix—Solid Lubricant Composites. In CRC Handbook of Lubrication and Tribology, Volume III, Monitoring, Materials, Synthetic Lubricants, and Applications; Booser, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Merstallinger, A.; Simon, Z.; Nuss, G.; Paul, C.E.; Palladino, M.; Buttery, M. New Self Lubricating Polymer Matrix Composites for Journal and Ball Bearing Applications in Space (SLPMC2). In Proceedings of the 17th European Space Mechanisms & Tribology Symposium—ESMATS 2017, Hatfield, UK, 20–22 September 2017; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2017/merstallinger.pdf (accessed on 15 February 2020).
- Sobey, A.R.; Lockett, T.R. Design and Development of NEA Scout Solar Sail Deployer Mechanism. In Proceedings of the 43rd Aerospace Mechanisms Symposium, Santa Clara, CA, USA, 4–6 May 2016; Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2016/sobey.pdf (accessed on 15 February 2020).
- Lancaster, J.K. Dry bearings: A survey of materials and factors affecting their performance. Tribology 1973, 6, 219–251. [Google Scholar] [CrossRef]
- A Guide on the Design and Selection of Dry Rubbing Bearings; Item 76209; Engineering Sciences Data Unit: London, UK, 1976.
- Gould, S.G.; Roberts, E.W. The In-Vacuo Torque Performance of Dry-Lubricated Ball Bearings at Cryogenic Temperatures. In Proceedings of the 23th Aerospace Mechanisms Symposium, Huntsville, AL, USA, 3–5 May 1989; p. 319. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19890014543.pdf (accessed on 15 February 2020).
- Wyn-Roberts, D. ESTL Progress Report for 1986; Report No. ESA (ESTL) 79; ESA: Warrington, Cheshire, UK, 1987. [Google Scholar]
- Barber, S.A.; Kannel, J.W.; Dufrane, K.F. Evaluation of Transfer Films of Salox M on 440C for HPOTP Bearing Cage Applications. Battelle; NASA-CR-179020; Battelle: Columbus, OH, USA, 1986. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19880019800.pdf (accessed on 15 February 2020).
- Nishimura, M.; Seki, K.; Miyakawa, Y. Lubrication Characteristics of Rolling Bearings Lubricated by Self-Lubricating Composite Retainers (Part I) - Selection of Retainers for Earth Sensors. In Proceedings of the 18th International Symposium on Space Technology and Science, Kagoshima, Japan, 17–22 May 1992; p. 547. [Google Scholar]
- Gill, S. A Comparison of the Performance of Solid and Liquid Lubricants in Oscillating Spacecraft Ball Bearings. In Proceedings of the 28th Aerospace Mechanisms Symposium, Cleveland, OH, USA, 18–20 May 1994; p. 229. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028805.pdf (accessed on 15 February 2020).
- Soriano, B.L.; Gardos, M.N.; Buller, B.W. Polyimide Composite Retainer Materials Optimized for Minimal Wear and Film Transfer. Tribol. Trans. 1993, 36, 670. [Google Scholar] [CrossRef]
- Suzuki, M.; Nishimura, M. Tribological Characteristics of Ball Bearings Lubricated with a Sputtered Molybdenum Disulphide Film in a Vacuum under a High Thrust Load. In Proceedings of the 5th European Space Mechanisms and Tribology Symposium (ESMATS), ESTEC, Noordwijk, The Netherlands, 28–30 October 1992. [Google Scholar]
- Anderson, M.J. Ball bearing tests to evaluate Duroid replacements. In Proceedings of the 9th European Space Mechanisms and Tribology Symposium, Liège, Belgium, 19–21 September 2001; pp. 43–47. Available online: http://articles.adsabs.harvard.edu/pdf/2001ESASP.480...43A (accessed on 15 February 2020).
- Kreuser, J.; Bachmann, R.; Bergrath, B.; Heinrich, B.; Zemann, J. Development of Motor Bearings for a New SADA (Bepi Colombo). In Proceedings of the 15th European Space Mechanisms and Tribology Symposium (ESMATS 2013), Noordwijk, The Netherlands, 25–27 September 2013; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2013/kreuser.pdf (accessed on 15 February 2020).
- Colas, G.; Saulot, A.; Descartes, S.; Michel, Y.; Berthier, Y. Double Transfer Experiments to Highlight Design Criterion for Future Self-Lubricating Materials. In Proceedings of the 16th European Space Mechanisms and Tribology Symposium (ESMATS 2015), Bilbao, Spain, 23–25 September 2015; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2015/colas.pdf (accessed on 15 February 2020).
- Thomson, M.W. Deployable and Retractable Telescoping Tubular Structure Development. In Proceedings of the 28th Aerospace Mechanisms Symposium, Cleveland, OH, USA, 18–20 May 1994; p. 323. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19940028811.pdf (accessed on 15 February 2020).
- Kuhn, A. PTFE Coating vs. Impregnation – Analyzing what works best for anodized aluminum. Metal Finish. 2005, 103, 35–38. [Google Scholar] [CrossRef]
- Rabinowicz, E. Friction coefficients of noble metals over a range of loads. Wear 1992, 159, 89. [Google Scholar] [CrossRef]
- Karassik, I.J. (Ed.) Pump Handbook; Section 5.1; Metallic Materials of Pump Construction; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Colas, G.; Saulot, A.; Michel, Y.; Baudasse, Y.; Mistral, A.; Berthier, Y. Dry Lubrication Efficiency: From Ground to Space. In Proceedings of the 15th European Space Mechanisms and Tribology Symposium (ESMATS 2013), Noordwijk, The Netherlands, 25–27 September 2013; Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2013/colas.pdf (accessed on 15 February 2020).
- Matsumoto, K.; Suzuki, M. Tribological Performance of Sputtered MoS2 Films in Various Environment—Influence of oxygen concentration, water vapor and gas species. In Proceedings of the 8th European Space Mechanisms & Tribology Symposium—ESMATS 1999, Toulouse, France, 29 September–1 October 1999; p. 43. Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/1999/matsumoto.pdf (accessed on 15 February 2020).
- Söchting, S.; Sherrington, I.; Lewis, S.D.; Roberts, E.W. An evaluation of the effect of simulated launch vibration on the friction performance and lubrication of ball bearings for space applications. Wear 2006, 260, 1190–1202. [Google Scholar] [CrossRef]
- Report of the SEASAT Failure Review Board; NASA CASI No. N93-24693; NASA: Washington, DC, USA, 1993. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19930015504.pdf (accessed on 15 February 2020).
- Peabody, S.A. Design and Requirements Creep in a Build-To-Print Mission. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, SC, USA, 16–20 July 2017; Available online: https://ttu-ir.tdl.org/bitstream/handle/2346/73094/ICES_2017_326.pdf (accessed on 15 February 2020).
- Buttery, M.; Lewis, S.; Kent, A.; Bingley, R.; Cropper, M. Long-Term Storage Considerations for Spacecraft Lubricants. Lubricants 2020, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Kimsey, B.C. Terrestrial Satellite Storage. In Institute of Environmental Sciences, 11th Test Seminar; IES: Manhattan Beach, CA, USA, 1988; p. 203. [Google Scholar]
- Grattan, P.A.; Lancaster, J.K. Abrasion by Lamellar Solid Lubricants. Wear 1967, 10, 453. [Google Scholar] [CrossRef]
- Electrostatic Discharge Control Handbook (Program) for Protection of Electrical and Electronic Parts, Assemblies, and Equipment (excluding Electrically Initiated Explosive Devices); Military Handbook MIL HDBK 263A, 22 February 1991; Military Standard MIL STD 1686A, Defense Quality and Standardization Office: Falls Church, VA, USA, 8 August 1988.
- Stewart, T.B.; Fleischauer, P.D. Chemistry of Sputtered Molybdenum Disulfide Films. Inorg. Chem. 1982, 21, 2426. [Google Scholar] [CrossRef]
- Jayaram, G.; Marks, L.D.; Hilton, M.R. Nanostructure of Au-20%Pd Layers in Sputter-Deposited MoS2 Films. Surf. Coat. Technol. 1995, 76–77 Pt 2, 393–399. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Iijima, T.; Kaneko, Y.; Miyakawa, Y.; Nishimura, M. Tribological characteristics of bonded MoS2 films evaluated in rolling-sliding contact in a vacuum. Wear 1995, 190, 219–225. [Google Scholar] [CrossRef]
- Veinot, D.E.; Langille, K.B.; Nguyen, D.T.; Bernt, J.O. Efflorescence of soluble silicate coatings. J. Non-Cryst. Sol. 1991, 127, 221–226. [Google Scholar] [CrossRef]
- Peterson, M.B.; Johnson, R.L. Friction and Wear Characteristics of Molybdenum Disulfide. I: Effect of Moisture; NACA Tech. Note NACA TN 3055, NACA: Washington, DC, USA, 1953. [Google Scholar]
- Fusaro, R.L. Lubrication and Failure Mechanisms of Molybdenum Disulfide Films: Part I—Effect of Atmosphere; NASA Tech Paper 1343; NASA Lewis Research Ctr: Cleveland, OH, USA, 1978.
- Hiraoka, N. Wear life mechanism of journal bearings with bonded MoS2 film lubricants in air and vacuum. Wear 2002, 249, 1014–1020. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. The Effects of Environmental Water and Oxygen on the Temperature-Dependent Friction of Sputtered Molybdenum Disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Key, C.F.; Riehl, W.A. Compatibility of Materials with Liquid Oxygen; NASA TM X-985; NASA: Washington, DC, USA, 1964. Available online: https://ntrs.nasa.gov/search.jsp?R=19750065862 (accessed on 15 February 2020).
- Yen, B.K.; Schwickert, B.E. Origin of low-friction behavior in graphite investigated by surface x-ray diffraction. Appl. Phys. Lett. 2004, 84, 4702–4704. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, J.K.; Pritchard, J.R. The influence of environment and pressure on the transition to dusting wear of graphite. J. Phys. D Appl. Phys. 1981, 14, 747–762. [Google Scholar] [CrossRef]
- Braithwaite, E.R. Friction and Wear of Graphite and Molybdenum Disulfide, Pt. 2. Sci. Lubr. 1966, 18, 17. [Google Scholar] [CrossRef]
- Savage, R.H.; Schaefer, D.L. Vapor Lubrication of Graphite Sliding Contacts. J. Appl. Phys. 1956, 27, 136. [Google Scholar] [CrossRef]
- Lubricant, Solid Film, Heat Cured, Corrosion Inhibiting, Procurement Specification; AS5272E; SAE International: Warrendale, PA, USA, 2016; Available online: https://www.sae.org/standards/content/as5272e/ (accessed on 15 February 2020).
- Karapetyan, S.S.; Silin, A.A. Temperature Dependence of the Coefficient of Friction of Molybdenum Disulfide in a Vacuum from 300 to 80 K. Sov. Phys. Dokl. 1976, 21, 390–391. [Google Scholar]
- Zhao, X.; Phillpot, S.R.; Sawyer, W.G.; Sinnott, S.B.; Perry, S.S. Transition from Thermal to Athermal Friction under Cryogenic Conditions. Phys. Rev. Lett. 2009, 102, 186102. [Google Scholar] [CrossRef] [Green Version]
- Lince, J.R.; Kim, H.I.; Kirsch, J.J.; Didziulis, S.V. Low-Temperature Friction Variation of MoS2-Based Lubricants. Proceedongs of the Presented at the 71st STLE Annual Meeting & Exhibition, Las Vegas, NV, USA, 15–19 May 2016; Available online: https://www.researchgate.net/publication/317006534_Low-Temperature_Friction_Variation_of_MoS2-Based_Lubricants (accessed on 15 February 2020).
- Burris, D.L.; Perry, S.S.; Sawyer, W.G. Macroscopic Evidence of Thermally Activated Friction with Polytetrafluoroethylene. Tribol. Lett. 2007, 27, 323–328. [Google Scholar] [CrossRef]
- Serles, P.; Gaber, K.; Pajovic, S.; Colas, G.; Filleter, T. High Temperature Microtribological Studies of MoS2 Lubrication for Low Earth Orbit. Lubricants 2020, 8, 49. [Google Scholar] [CrossRef]
- Arita, M.; Yasuda, Y.; Kishi, K.; Ohmae, N. Investigations of Tribological Characteristics of Solid Lubricants Exposed to Atomic Oxygen. Tribol. Trans. 1992, 35, 374. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Matsumoto, K.; Suzuki, M.; Teraoka, Y.; Kitamura, A.; Belin, M.; Fontaine, J.; Martin, J.-M. Space Environmental Effects on MoS2 and Diamond-like Carbon Lubricating Films: Atomic Oxygen-induced Erosion and its Effect on Tribological Properties. Surf. Coat. Technol. 2007, 202, 1003–1010. [Google Scholar] [CrossRef]
- Bauer, R.; Fleischauer, P. Torque Characteristics of Solid-Lubricated Precision Bearings During Oscillatory Motion. Trib. Trans. 1995, 38, 1–10. [Google Scholar] [CrossRef]
- Compostizo, C.; López, R.; Rivera, L. GAIA M2M Pointing Mechanism Qualification. In Proceedings of the 14th European Space Mechanisms & Tribology Symposium—SMATS 2011, Constance, Germany, 28–30 September 2011; pp. 211–218. Available online: http://esmats.eu/esmatspapers/pastpapers/pdfs/2011/compostizo.pdf (accessed on 15 February 2020).
- Merstallinger, A.; Semerad, E.; Dunn, B.D. Influence of Coatings and Alloying on Cold Welding due to Impact and Fretting. In Proceedings of the 10th European Space Mechanisms & Tribology Symposium—ESMATS 2011, San Sebastian, Spain, 24–26 September 2003; Available online: http://www.esmat.esa.int/Publications/Published_papers/Merstallinger_037_ColdWelding_color.pdf (accessed on 15 February 2020).
- Wood, J.P.; de la Fuente, J. Wear Life Testing of a Mission Critical Separation Interface. In Proceedings of the 39th Aerospace Mechanisms Symposium, Huntsville, AL, USA, 7–9 May 2008; pp. 157–170. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2008/wood.pdf (accessed on 15 February 2020).
- Phinney, D.D. Slip Ring Experience in Long-Term Space Applications; NASA CASI No. N87-16325; Ball Brothers Research Corp.: Boulder, CO, USA, 1986. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19870006892.pdf (accessed on 15 February 2020).
- Pitney, K.E. Ney Contact Manual; J.M. Ney Co.: Bloomfield, CT, USA, 1973; Available online: https://www.deringerney.com/publications/ney_electrical_contact_manual/ (accessed on 15 February 2020).
- Fiber Brush Slip Ring Technology. Available online: https://www.moog.com/content/dam/moog/literature/MCG/fiberbrush.pdf (accessed on 15 February 2020).
- Koss, S.; Woolaway, S. Lessons Learned from the Windsat BAPTA Design and On-Orbit Anomalies. In Proceedings of the 38th Aerospace Mechanisms Symposium, Langley Research Center, Hampton, VA, USA, 17–19 May 2006; Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2006/koss.pdf (accessed on 15 February 2020).
- Rottmeier, F.; Krummen, M.; Miler, M. Development of High Temperature High Current Contact Technology in Slipring Assemblies for the BepiColombo MPO & MTM Spacecrafts. In Proceedings of the 41st Aerospace Mechanisms Symposium, Pasadena, CA, USA, 16–18 May 2012; pp. 195–209. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2012/rottmeier.pdf (accessed on 15 February 2020).
- Lewis, N.E.; Cole, S.R.; Glossbrenner, E.W.; Vest, C.E. Friction, Wear, and Noise of Slip Ring and Brush Contacts for Synchronous Satellite Use. In Proceedings of the 1st European Space Mechanisms & Tribology Symposium, Frascati, Italy, 9–11 April 1975. [Google Scholar] [CrossRef]
- Glossbrenner, E.W.; Witherspoon, B.K. Brushes Containing Molybdenum Disulfide for Slip Rings in Spacecraft Application. In Proceedings of the 33rd IEEE Holm Conference on Electrical Contacts, Chicago, IL, USA, 21–23 September 1987; p. 253. [Google Scholar]
- Clauss, F.J.; Kingery, M.K. Sliding Electrical Contact Materials for Use in Ultrahigh Vacuum. In Proceedings of the 6th Structures and Materials Conference AIAA, Palm Springs, CA, USA, 4 April 1967. [Google Scholar] [CrossRef]
- Sambamoorthy, V.R.; Varghese, P.M. Development of Sliprings for Spacecraft Application. In Proceedings of the National Conferemce: Indus Tribology (NCIT-89), Trivandrum, India, 19–20 January 1989; Volume 1, p. VII-2.1. [Google Scholar]
- Matteo, D.N. Improving Slip-Ring Performance. In Proceedings of the 18th Aerospace Mechanisms Symposium, Greenbelt, MD, USA, 2–4 May 1984; p. 111. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19840017018.pdf (accessed on 15 February 2020).
- Pentlicki, C.J.; Glossbrenner, E.W. The Testing of Contact Materials for Slip Rings and Brushes for Space Application. In Electrical Contacts-1971 (Proc. Holm Seminar Elec. Contact Phenom.); Illinois Institute of Technology: Chicago, Illinois, USA, 1971; p. 157. [Google Scholar]
- Bauer, R. Sulfide Corrosion of Silver Contacts During Satellite Storage. J. Spacecr. Rockets 1988, 25, 439. [Google Scholar] [CrossRef]
- Roberts, E.W. Observations of High Resistance on Slip-Rings Employing Ag/MoS2/Cu Brushes (Interim Report); ESA Contract Report No. ESA(ESTL)70; ESA: Warrington, Cheshire, UK, 1984. [Google Scholar]
- Gleeson, J.; Dufrane, K.; Kannel, J. Replacement Bearing for Rocketdyne SSME HPOPTPs using Alternate Self-Lubricating Retainer Materials; NASA Report NASA-CR-184328; Battelle: Columbus, OH, USA, 1992. Available online: https://ntrs.nasa.gov/search.jsp?R=19920019119 (accessed on 15 February 2020).
- Gibson, H. Lubrication of Space Shuttle Main Engine Turbopump Bearings. Lubr. Eng. 2001, 57, 10–12. [Google Scholar]
- Grygorczuk, J.; Wiśniewski, L.; Kędziora, B.; Borys, M.; Przybyła, R.; Kuciński, T.; Ossowski, M.; Konior, W.; Krömer, O.; Spohn, T.; et al. Hammering Mechanism for HP3 Experiment (InSight). In Proceedings of the 43rd Aerospace Mechanisms Symposium, Santa Clara, CA, USA, 4–6 May 2016; Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2016/grygorczuk2.pdf (accessed on 15 February 2020).
- Erdemir, A.; Donnet, C. Tribology of diamond-like carbon films: Recent progress and future prospects. J. Phys. D Appl. Phys. 2006, 39, R311–R327. [Google Scholar] [CrossRef]
- Jiang, J. The effect of relative humidity on wear of a diamond-like carbon coating. Surf. Coat. Technol. 2003, 167, 221–225. [Google Scholar] [CrossRef]
- Kim, H.I.; Lince, J.R.; Eryilmaz, O.L.; Erdemir, A. Environmental effects on the friction of hydrogenated DLC films. Trib. Lett. 2006, 21, 53–58. [Google Scholar] [CrossRef]
- Vanhulsel, A.; Velasco, F.; Jacobs, R.; Eersels, L.; Havermans, D.; Roberts, E.W.; Sherrington, I.; Anderson, M.J.; Gaillard, L. DLC solid lubricant coatings on ball bearings for space applications. Trib. Int. 2007 40, 1186–1194. [CrossRef]
- Lince, J.R. Tribology in the Space Environment. In Proceedings of the Presented at the 2015 International Conference on Metallurgical Coatings & Thin Films (ICMCTF), San Diego, CA, USA, 23 April 2015. [Google Scholar]
- Kalin, M.; Velkayrh, I.; Vižintin, J.; Ožbolt, L. Review of boundary lubrication mechanisms of DLC coatings used in mechanical applications. Meccanica 2008, 43, 623–637. [Google Scholar] [CrossRef]
- Lince, J.R.; Bertrand, P.A.; Eryilmaz, O.L.; Erdemir, A. Tribochemistry of Multiply-Alkylated Cyclopentane Oils on DLC-Coated Thrust Bearings. J. ASTM Int. 2008, 5, JAI101223. [Google Scholar] [CrossRef]
- Conley, P.L.; Bohner, J.J. Experience with Synthetic Fluorinated Fluid Lubricants. In Proceedings of the 24th Aerospace Mechanisms Symposium, Kennedy Space Center, FL, USA, 18–20 April 1990; pp. 213–230. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900012779.pdf (accessed on 15 February 2020).
- Fontaine, J. Towards the use of diamond-like carbon solid lubricant coatings in vacuum and space environments. Proc. IMechE Part J J. Eng. Tribol. 2008, 222, 1015–1029. [Google Scholar] [CrossRef]
- Miyoshi, K.; Murakawa, M.; Watanabe, S.; Takeuchi, S.; Wu, R.L.C. Tribological Characteristics and Applications of Superhard Coatings: CVD Diamond, DLC, and c-BN. In Proceedings of the Applied Diamond Conference/Frontier Carbon Technology Joint Conference 1999, Tsukuba, Japan, 31 August–3 September 1999; pp. 268–273. Available online: https://ntrs.nasa.gov/search.jsp?R=19990081119 (accessed on 15 February 2020).
- Watanabe, S.; Wheeler, D.R.; Abel, P.B.; Street, K.W.; Miyoshi, K.; Murakawa, M.; Miyake, S. Surface Chemistry, Microstructure, and Tribological Properties of Cubic Boron Nitride Films; NASA-TM-113163; NASA Lewis Research Ctr.: Cleveland, OH, USA, 1998. Available online: https://ntrs.nasa.gov/search.jsp?R=19980016167 (accessed on 15 February 2020).
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to optimized Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Johnson, J.A.; Woodford, J.B.; Chen, X.; Andersson, J.; Erdemir, A.; Fenske, G.R. Insights into Near-Frictionless Carbon Films. J. Appl. Phys. 2004, 95, 7765–7771. [Google Scholar] [CrossRef]
- Hua, X.; Sun, J.; Zhang, P.; Liu, K.; Wang, R.; Ji, J.; Fu, Y. Tribological Properties of Laser Microtextured Surface Bonded with Composite Solid Lubricant at High Temperature. J. Tribol. 2016, 138, 031302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voevodin, A.A.; Zabinski, J.S. Laser surface texturing for adaptive solid lubrication. Wear 2006, 261, 1285–1292. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Chameleon Coatings: Adaptive Surfaces to Reduce Friction and Wear in Extreme Environments. Ann. Rev. Mater. Res. 2009, 39, 297–324. [Google Scholar] [CrossRef] [Green Version]
- Voevodin, A.A.; O’Neil, J.P.; Zabinski, J.S. WC/DLC/WS2 nanocomposite coatings for aerospace tribology. Tribol. Lett. 1999, 6, 75–78. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Fitz, T.A.; Hu, J.J.; Zabinski, J.S. Nanocomposite tribological coatings with “chameleon” surface adaptation. J. Vac. Sci. Technol. A 2002, 20, 1434. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A.; Hu, J.J.; Zabinski, J.S. Tribology of adaptive nanocomposite yttria-stabilized zirconia coatings containing silver and molybdenum. Wear 2006, 261, 797–805. [Google Scholar] [CrossRef]
- Magnéli, A. Structures of the ReOx-type with recurrent dislocations of homologous series of molybdenum and tungsten oxides. Acta. Crystallogr. 1953, 6, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Fateh, N.; Fontalvo, G.A.; Gassner, G.; Mitterer, C. The beneficial effect of high-temperature oxidation on the tribological behavior of V and VN coatings. Tribol. Lett. 2007, 28, 1–7. [Google Scholar] [CrossRef]
- Muratore, C.; Hu, J.J.; Voevodin, A.A. Adaptive nanocomposite coatings with a titanium nitride diffusion barrier mask for high-temperature tribological applications. Thin Solid Films 2007, 515, 3638–3643. [Google Scholar] [CrossRef]
- Buttery, M.; Kent, A.; Forster, D.; Vortselas, A. Hybrid Lubrication of PFPE Fluids and Sputtered MoS2. In Proceedings of the 44th Aerospace Mechanisms Symposium, Cleveland, OH, USA, 16–18 May 2018; pp. 151–164. Available online: http://esmats.eu/amspapers/pastpapers/pdfs/2018/buttery.pdf (accessed on 15 February 2020).
- Jones, W.R.; Pepper, S.V.; Jansen, M.J.; Nguyen, Q.N.; Kingsbury, E.P.; Loewenthal, S.H.; Predmore, R.E. A New Apparatus to Evaluate Lubricants for Space Applications—The Spiral Orbit Tribometer (SOT); NASA/TM–2000-209935; NASA Glenn Research Ctr.: Cleveland, OH, USA, 2000. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20000029564.pdf (accessed on 15 February 2020). [CrossRef] [Green Version]


















| Property | Solid Lubricants | Liquid Lubricants |
|---|---|---|
| Volatility/Migration | Negligible volatility/Debris migration possible | Finite vapor pressure/Confinement required |
| Endurance | Life determined by lube wear—resupply difficult | Resupply possible—longer life |
| Temperature Range | Wide operating temperatures | Physical properties vary significantly with temperature |
| Friction/Torque Noise | Friction sensitive to debris → torque noise | Low torque noise—good models for torque calculation |
| Possibility of Accelerated Testing | Accelerated testing possible if failure mechanism known | Accelerated testing difficult |
| Air/Moisture Sensitivity | Life/friction can be air/moisture sensitive | Less sensitive to air/moisture |
| Electrical Conductivity | Electrically conducting | Poor electrical conductors |
| Substrate Sensitivity | Good adhesion is critical | Additives need to be matched to substrate |
| Lubricant Coating Formulation a | Property | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Hertzian Contact Stress Limit (Sm, ksi) b | Friction, in Vacuum | Approx. Thick-Ness | Thickness Variation | Min. Temp. (°C) | Max. Temp. (°C) | Adhesion | LOX Compatibility | Sliding/Rolling (S/R) c | Relative Endurance | Robustness to Humid Air Storage | Resistance to Condensed Moisture | |
| Unbonded MoS2 or WS2 | 200 | 0.02–0.1 | 0.1–10 µm | ±80% | −260 | 900 (in vacuum) | Low | Yes | S/(R) | Very Low | Fair | Fair |
| Unbonded PTFE | 2 | 0.02–0.2 | 1 µm | ±80% | −35 | 150 to 250 | Low | Yes | S/(R) | Low | Very good | Very good |
| Resin-bonded/Heat Cured | 100 | 0.03–0.1 | 10 µm | ±50% | −220 to −70 | 200 to 400 | Medium | No | S/(R) | High | Very good | Good |
| Resin-bonded/Air Cured | 50 | 0.03–0.1 | 10 µm | ±50% | −220 to −70 | 150 to 400 | Medium | No | S/(R) | Medium | Good | Fair |
| Inorganic-bonded | 150 | 0.03–0.2 | 10 µm | ±50% | −250 to −70 | 370 to 850 | Medium | Yes | S/(R) | Medium | Fair | Poor |
| Ceramic-bonded | 100 | 0.1–0.2 | 10 µm | ±50% | −240 to 20 | 600 to 1100 | Medium | Yes | S/(R) | Medium | Fair | Poor |
| Sputter-Deposited MoS2 | 200 | 0.003–0.05 | 1 µm | ±10% | −260 | 400 | High | Yes | S/R | High | Fair/Good e | Fair/Good e |
| Ion Plated Pb | 130 | 0.1–0.3 | 0.1–1 µm | ±10% | −260 | 300 | High | Yes | (S)/R | Medium | Fair | Fair |
| Carbide/Nitride Coatings | >300 | 0.4–0.8 | 0.1–2 µm | ±10% | Varies | >700 | High | Yes | (S)/R | High | Very good | Very good |
| HH-DLC | >200 | 0.001–0.01 | 1 µm | ±10% | < −200 | 300 | High | Yes | S/R | High | Very good | Very good |
| Bulk Lubricating Materials d | 1–50 | 0.02–0.4 | ~1 cm | N/A | Varies | Varies | N/A | Yes | S/R | High | Varies | Varies |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lince, J.R. Effective Application of Solid Lubricants in Spacecraft Mechanisms. Lubricants 2020, 8, 74. https://doi.org/10.3390/lubricants8070074
Lince JR. Effective Application of Solid Lubricants in Spacecraft Mechanisms. Lubricants. 2020; 8(7):74. https://doi.org/10.3390/lubricants8070074
Chicago/Turabian StyleLince, Jeffrey R. 2020. "Effective Application of Solid Lubricants in Spacecraft Mechanisms" Lubricants 8, no. 7: 74. https://doi.org/10.3390/lubricants8070074
APA StyleLince, J. R. (2020). Effective Application of Solid Lubricants in Spacecraft Mechanisms. Lubricants, 8(7), 74. https://doi.org/10.3390/lubricants8070074