Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Bees and Rearing Conditions
2.2. N. ceranae Spores
2.3. Infection Experiments: Group A and Group B
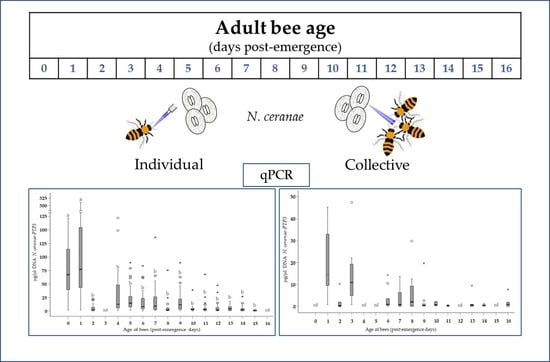
2.3.1. Group A: Individual Infection of Bees at Different Ages
2.3.2. Group B: Collective Infection of Groups of Bees at Different Ages
2.4. Molecular Detection of N. ceranae Infection (DNA Extraction and qPCR Analysis)
2.5. Statistical Analysis: N. ceranae Infection According to Bee Age and the Mode of Infection
3. Results
3.1. Level of Infection in Bees of Different Ages (Group A)
3.2. Level of Infection According to Age and Method of Infection (Group A vs. Group B)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarez-Suarez, J.M. Bee Products-Chemical and Biological Properties; Facultad de Ciencias de la Salud, Universidad de Las Americas, Ed.; Springer International Publishing AG: Quito, Ecuador, 2017; ISBN 9783319596891. [Google Scholar]
- European Commission. EU Pollinators Initiative: Roadmap; European Commission: Brussels, Belgium, 2017; pp. 1–3. [Google Scholar]
- IPBES. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Potts, S.G., Imperatriz-Fonseca, V.L., Ngo, H.T., Biesmeijer, J.C., Breeze, T.D., Dicks, L.V., Garibaldi, L.A., Hill, R., Settele, J., Vanbergen, A.J., et al., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; 36p, ISBN 978-92-807-3567-3. [Google Scholar]
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 2018, 49, 131–150. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, Y.S.; Zinatullina, Z.Y.; Ignatieva, A.N.; Zhigileva, O.N.; Malysh, J.M.; Sokolova, Y.Y. Detection of two Microsporidia pathogens of the European honey bee Apis mellifera (Insecta: Apidae) in Western Siberia. Acta Parasitol. 2018, 63, 728–732. [Google Scholar] [CrossRef]
- Ostroverkhova, N.V.; Kucher, A.N.; Golubeva, E.P.; Rosseykina, S.A.; Konusova, O.L. Study Of Nosema spp. In The Tomsk Region, Siberia: Co-Infection Is Widespread In Honeybee Colonies. Far East. Entomol. 2019, 378, 12–22. [Google Scholar] [CrossRef]
- Gisder, S.; Schüler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-Term Temporal Trends of Nosema spp. Infection Prevalence in Northeast Germany: Continuous Spread of Nosema ceranae, an Emerging Pathogen of Honey Bees (Apis mellifera), but No General Replacement of Nosema apis. Front. Cell. Infect. Microbiol. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years post-detection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Garrido-Bailón, E.; González-Porto, A.; Martínez-Salvador, A.; De La Rúa, P.; Meana, A.; Higes, M. The growing prevalence of Nosema ceranae in honey bees in Spain, an emerging problem for the last decade. Res. Vet. Sci. 2012, 93, 150–155. [Google Scholar] [CrossRef]
- Cepero, A.; Martín-Hernández, R.; Bartolomé, C.; Gómez-Moracho, T.; Barrios, L.; Bernal, J.; Teresa Martín, M.; Meana, A.; Higes, M. Passive laboratory surveillance in Spain: Pathogens as risk factors for honey bee colony collapse. J. Apic. Res. 2016, 54, 525–531. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F. Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J. Invertebr. Pathol. 2013, 113, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE 2012, 7, e43319. [Google Scholar] [CrossRef] [PubMed]
- Webster, T. Nosema apis spore transmission among honey bees. Am. Bee J. 1993, 133, 869–870. [Google Scholar]
- Higes, M.; Martin-Hernandez, R.; Garcia-Palencia, P.; Martin, P.; Meana, A.M. Horizontal transmission of Nosema ceranae (Microsporidia) from worker honeybees to queens (Apis mellifera). Environ. Microbiol. Rep. 2009, 1, 495–498. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- García-Palencia, P.; Martín-Hernández, R.; González-Porto, A.-V.; Marin, P.; Meana, A.; Higes, M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J. Apic. Res. 2010, 49, 278–283. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae Tissue Tropism in Worker Honey Bees (Apis mellifera). Vet. Pathol. 2019. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.H.; Martín-Hernández, R.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Doublet, V.; Natsopoulou, M.E.; Zschiesche, L.; Paxton, R.J. Within-host competition among the honey bees pathogens Nosema ceranae and Deformed wing virus is asymmetric and to the disadvantage of the virus. J. Invertebr. Pathol. 2015, 124, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.F.; Solter, L.; Aronstein, K.; Huang, Z. Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 2015, 124, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.S.; Evans, J.D. Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef]
- Williams, G.R.; Shutler, D.; Burgher-MacLellan, K.L.; Rogers, R.E.L. Infra-population and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS ONE 2014, 9, 5–10. [Google Scholar] [CrossRef]
- Vidau, C.; Panek, J.; Texier, C.; Biron, D.G.; Belzunces, L.P.; Le Gall, M.; Broussard, C.; Delbac, F.; El Alaoui, H. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J. Invertebr. Pathol. 2014, 121, 89–96. [Google Scholar] [CrossRef]
- Gage, S.L.; Kramer, C.; Calle, S.; Carroll, M.; Heien, M.; DeGrandi-Hoffman, G. Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 2018, 221, jeb161489. [Google Scholar] [CrossRef]
- Wolf, S.; McMahon, D.P.; Lim, K.S.; Pull, C.D.; Clark, S.J.; Paxton, R.J.; Osborne, J.L. So near and yet so far: Harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE 2014, 9, e103989. [Google Scholar] [CrossRef]
- Lecocq, A.; Jensen, A.B.; Kryger, P.; Nieh, J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Fleites-Ayil, F.A.; Quezada-Euán, J.J.G.; Medina-Medina, L.A. Onset of foraging and lifespan of Africanized honey bees (Apis mellifera) infected with different levels of Nosema ceranae spores in Neotropical Mexico. Apidologie 2018, 49, 781–788. [Google Scholar] [CrossRef]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and Behavioral Changes in Honey Bees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Rinderer, T.E.; Sylvester, H.A.; Holloway, B.; Oldroyd, B.P. Patterns of Apis mellifera infestation by Nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie 2012, 43, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Fontbonne, R.; Garnery, L.; Vidau, C.; Aufauvre, J.; Texier, C.; Tchamitchian, S.; Alaoui, H.E.; Brunet, J.L.; Delbac, F.; Biron, D.G. Comparative susceptibility of three Western honeybee taxa to the microsporidian parasite Nosema ceranae. Infect. Genet. Evol. 2013, 17, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Giersch, T.; Berg, T.; Galea, F.; Hornitzky, M. Nosema ceranae infects honey bees ( Apis mellifera ) and contaminates honey in Australia. Apidologie 2009, 40, 117–123. [Google Scholar] [CrossRef] [Green Version]
- van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–9 and 2009–10. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Malone, L.A.; Gatehouse, H.S. Effects of Nosema apis Infection on Honey Bee (Apis mellifera) Digestive Proteolytic Enzyme Activity. J. Invertebr. Pathol. 1998, 71, 169–174. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Meana, A.; García-Palencia, P.; Marín, P.; Botías, C.; Garrido-Bailón, E.; Barrios, L.; Higes, M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009, 75, 2554–2557. [Google Scholar] [CrossRef] [Green Version]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [Green Version]
- Fenoy, S.; Rueda, C.; Higes, M.; Martín-Hernández, R.; Del Aguila, C. High-level resistance of Nosema ceranae, a parasite of the honeybee, to temperature and desiccation. Appl. Environ. Microbiol. 2009, 75, 6886–6889. [Google Scholar] [CrossRef] [Green Version]
- Tapaszti, Z.; Forgách, P.; Kővágó, C.; Békési, L.; Bakonyi, T.; Rusvai, M. First detection and dominance of Nosema ceranae in Hungarian honeybee colonies. Acta Vet. Hung. 2009, 57, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Meana, A.; Martín-Hernández, R.; Higes, M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. 2010, 49, 212–214. [Google Scholar] [CrossRef]
- Smart, M.D.; Sheppard, W.S. Nosema ceranae in age cohorts of the western honey bee (Apis mellifera). J. Invertebr. Pathol. 2012, 109, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.J.; Lucas, H.M.; Webster, T.C.; Sagili, R.R. Colony level prevalence and intensity of Nosema ceranae in honey bees (Apis mellifera L.). PLoS ONE 2016, 11, e0163522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Effects of host age on susceptibility to infection and immune gene expression in honey bee queens (Apis mellifera) inoculated with Nosema ceranae. Apidologie 2014, 45, 451–463. [Google Scholar] [CrossRef] [Green Version]
- VanEngelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.N.; Coleman, J.L.; Clinnin, K.; Fahrbach, S.; Rueppell, O. Age, caste, and behavior determine the replicative activity of intestinal stem cells in honeybees (Apis mellifera L.). Exp. Gerontol. 2008, 43, 530–537. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; Mcmahon, D.P.; Medrzycki, P.; et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Porrini, M.P.; Sarlo, E.G.; Medici, S.K.; Garrido, P.M.; Porrini, D.P.; Damiani, N.; Eguaras, M.J. Nosema ceranae development in Apis mellifera: Influence of diet and infective inoculum. J. Apic. Res. 2011, 50, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [Green Version]
- Martín-Hernández, R.; Botías, C.; Bailón, E.G.; Martínez-Salvador, A.; Prieto, L.; Meana, A.; Higes, M. Microsporidia infecting Apis mellifera: Coexistence or competition. Is Nosema ceranae replacing Nosema apis? Environ. Microbiol. 2012, 14, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Urbieta-Magro, A.; Higes, M.; Meana, A.; Gómez-Moracho, T.; Rodríguez-García, C.; Barrios, L.; Martín-Hernández, R. The levels of natural Nosema spp. infection in Apis mellifera iberiensis brood stages. Int. J. Parasitol. 2019, 49, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; García-Palencia, P.; Meana, A. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J. Invertebr. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; Botías, C.; García-Palencia, P.; Meana, A. Regurgitated pellets of Merops apiaster as fomites of infective Nosema ceranae (Microsporidia) spores. Environ. Microbiol. 2008, 10, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Taupin, V.; Méténier, G.; Vivarès, C.P.; Prensier, G. An improved procedure for Percoll gradient separation of sporogonial stages in Encephalitozoon cuniculi (Microsporidia). Parasitol. Res. 2006, 99, 708–714. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard methods for counting nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Forsgren, E.; Fries, I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef]
- Milbrath, M.O.; van Tran, T.; Huang, W.F.; Solter, L.F.; Tarpy, D.R.; Lawrence, F.; Huang, Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 2015, 125, 9–15. [Google Scholar] [CrossRef]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.; De la Mora, A.; Goodwin, P.H.; Habash, M.; Hamiduzzaman, M.M.; Kelly, P.G.; Guzman-Novoa, E. Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J. Microbiol. Methods 2016, 131, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Giacon, H.A.; Newton, M.R. Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L) to Nosema apis Z. Apidologie 1995, 26, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Gómez Moracho, T. Análisis de Los Patrones de Diversidad Genética de Nosema Ceranae, un Patógeno Emergente de Apis Mellifera; Universidade de Santiago de Compostela: Galicia, Spain, 2015. [Google Scholar]
- Suwannapong, G.; Yemor, T.; Boonpakdee, C.; Benbow, M.E. Nosema ceranae, a new parasite in Thai honeybees. J. Invertebr. Pathol. 2011, 106, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Eiri, D.M.; Suwannapong, G.; Endler, M.; Nieh, J.C. Nosema ceranae Can Infect Honey Bee Larvae and Reduces Subsequent Adult Longevity. PLoS ONE 2015, 10, e0126330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagastume, S.; Martín-Hernández, R.; Higes, M.; Henriques-Gil, N. Genotype diversity in the honey bee parasite Nosema ceranae: Multi-strain isolates, cryptic sex or both? BMC Evol. Biol. 2016, 16, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Bryden, J.; Paxton, R.J. Interspecific competition in honeybee intracellular gut parasites is asymmetric and favours the spread of an emerging infectious disease. Proc. R. Soc. B Biol. Sci. 2014, 282, 20141896. [Google Scholar] [CrossRef] [Green Version]
- Fries, I. Infectivity and Multiplication of Nosema apis Z. in the Ventriculus of the Honey Bee. Apidologie 1988, 19, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S.; Vanengelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Stear, M.J. OIE Nosemosis of Honey Bess. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Cambridge University Press: Cambridge, UK, 2018; pp. 744–749. [Google Scholar]
- Roberts, K.E.; Hughes, W.O.H. Immunosenescence and resistance to parasite infection in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2014, 121, 1–6. [Google Scholar] [CrossRef]
- Powell, J.E.; Martinson, V.G.; Urban-Mead, K.; Moran, N.A. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 2014, 80, 7378–7387. [Google Scholar] [CrossRef] [Green Version]
- Engel, P.; Bartlett, K.D.; Moran, N.A. The bacterium Frischella perrara causes scab formation in the gut of its honeybee host. MBio 2015, 6, e00193-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.E.; Rodrigues, P.A.P.; Mott, B.M.; Maes, P.; Corby-Harris, V. Ecological Succession in the Honey Bee Gut: Shift in Lactobacillus Strain Dominance During Early Adult Development. Microb. Ecol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hroncova, Z.; Havlik, J.; Killer, J.; Doskocil, I.; Tyl, J.; Kamler, M.; Titera, D.; Hakl, J.; Mrazek, J.; Bunesova, V.; et al. Variation in Honey Bee Gut Microbial Diversity Affected by Ontogenetic Stage, Age and Geographic Location. PLoS ONE 2015, 10, e0118707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, P.W.; Rodrigues, P.A.P.; Oliver, R.; Mott, B.M.; Anderson, K.E. Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol. Ecol. 2016, 25, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Rubanov, A.; Russell, K.A.; Rothman, J.A.; Nieh, J.C.; McFrederick, Q.S. Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci. Rep. 2019, 9, 3820. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Paleolog, J.; Borsuk, G. NosemaceranaeInfection Promotes Proliferation of Yeasts in Honey Bee Intestines. PLoS ONE 2016, 11, e0164477. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.E.; Ricigliano, V.A. Honey bee gut dysbiosis: A novel context of disease ecology. Curr. Opin. Insect Sci. 2017, 22, 125–132. [Google Scholar] [CrossRef]
- Regan, T.; Barnett, M.W.; Laetsch, D.R.; Bush, S.J.; Wragg, D.; Budge, G.E.; Highet, F.; Dainat, B.; de Miranda, J.R.; Watson, M.; et al. Characterisation of the British honey bee metagenome. Nat. Commun. 2018, 9, 4995. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Evans, J.D.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 2017, 12, e0187505. [Google Scholar] [CrossRef]
- Jones, J.C.; Fruciano, C.; Hildebrand, F.; Al Toufalilia, H.; Balfour, N.J.; Bork, P.; Engel, P.; Ratnieks, F.L.; Hughes, W.O. Gut microbiota composition is associated with environmental landscape in honey bees. Ecol. Evol. 2018, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.E.; Ricigliano, V.A.; Mott, B.M.; Copeland, D.C.; Floyd, A.S.; Maes, P. The queen’s gut refines with age: Longevity phenotypes in a social insect model. Microbiome 2018, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, L.; Williams, B.A.P.; Bursac, D.; Lithgow, T.; Keeling, P.J. Microsporidian mitosomes retain elements of the general mitochondrial targeting system. Proc. Natl. Acad. Sci. USA 2006, 103, 15916–15920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornman, R.S.; Chen, Y.P.; Schatz, M.C.; Street, C.; Zhao, Y.; Desany, B.; Egholm, M.; Hutchison, S.; Pettis, J.S.; Lipkin, W.I.; et al. Genomic analyses of the microsporidian Nosema ceranae, an emergent pathogen of honey bees. PLoS Pathog. 2009, 5, e1000466. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K.; Leonhard, B. Amino acids in honeybee worker haemolymph. Amino Acids 1997, 13, 141–153. [Google Scholar] [CrossRef]
- Weidner, E.; Findley, A.M.; Dolgikh, V.; Sokolova, J. Microsporidian Biochemistry and Physiology. In The Microsporidia and Microsporidiosis; Wittne, M., Weiss, L.M., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 172–195. ISBN 9781555818227. [Google Scholar]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef] [Green Version]
- Martín-Hernández, R.; Higes, M.; Sagastume, S.; Juarranz, Á.; Dias-Almeida, J.; Budge, G.E.; Meana, A.; Boonham, N. Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS ONE 2017, 12, e0170183. [Google Scholar] [CrossRef]
- Kurze, C.; Le Conte, Y.; Kryger, P.; Lewkowski, O.; Müller, T.; Moritz, R.F.A. Infection dynamics of Nosema ceranae in honey bee midgut and host cell apoptosis. J. Invertebr. Pathol. 2018, 154, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kurze, C.; Le Conte, Y.; Dussaubat, C.; Erler, S.; Kryger, P.; Lewkowski, O.; Müller, T.; Widder, M.; Moritz, R.F.A. Nosema Tolerant Honeybees (Apis mellifera) Escape Parasitic Manipulation of Apoptosis. PLoS ONE 2015, 10, e0140174. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Li, W.; Evans, J.D.; Li, J.; Su, S.; Hamilton, M.; Chen, Y. Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol. Res. 2017, 116, 3265–3274. [Google Scholar] [CrossRef]
- Holt, H.; Villar, G.; Grozinger, C.M. Molecular, physiological and behavioral responses of honey bee (Apis mellifera) drones to infection with microsporidian parasites. J. Invertebr. Pathol. 2018, 155, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Stroeymeyt, N.; Casillas-Pérez, B.; Cremer, S. Organisational immunity in social insects. Curr. Opin. Insect Sci. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Baracchi, D.; Cini, A. A Socio-Spatial Combined Approach Confirms a Highly Compartmentalised Structure in Honeybees. Ethology 2014, 120, 1167–1176. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; McMahon, D.P.; Paxton, R.J. Parasites modulate within-colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav. Ecol. Sociobiol. 2016, 70, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Naug, D.; Gibbs, A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 2009, 40, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Naug, D. Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav. Ecol. Sociobiol. 2008, 62, 1719–1725. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Moroń, D. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insectes Soc. 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Benvau, L.R.; Nieh, J.C. Larval honey bees infected with Nosema ceranae have increased vitellogenin titers as young adults. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography-mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef]
- Holt, H.L.; Aronstein, K.A.; Grozinger, C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genom. 2013, 14, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panek, J.; Paris, L.; Roriz, D.; Mone, A.; Dubuffet, A.; Delbac, F.; Diogon, M.; EI Alaoui, H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J. Invertebr. Pathol. 2018, 159, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pipan, N.; Rakovec, V. Cell Death in the Midgut Epithelium of the Worker Honey Bee (Apis mellifera carnica) During Metamorphosis. Zoomorphologie 1980, 94, 217–224. [Google Scholar] [CrossRef]
- Brodschneider, R.; Libor, A.; Kupelwieser, V.; Crailsheim, K. Food consumption and food exchange of caged honey bees using a radioactive labelled sugar solution. PLoS ONE 2017, 12, e0174684. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; VanEngelsdorp, D. Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef]
- Biganski, S.; Kurze, C.; Müller, M.Y.; Moritz, R.F.A. Social response of healthy honeybees towards Nosema ceranae-infected workers: Care or kill? Apidologie 2018, 49, 325. [Google Scholar] [CrossRef] [Green Version]
- Blacquière, T.; Smagghe, G.; Van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [Green Version]
- De Smet, L.; Hatjina, F.; Ioannidis, P.; Hamamtzoglou, A.; Schoonvaere, K.; Francis, F.; Meeus, I.; Smagghe, G.; De Graaf, D.C. Stress indicator gene expression profiles, colony dynamics and tissue development of honey bees exposed to sub-lethal doses of imidacloprid in laboratory and field experiments. PLoS ONE 2017, 12, e0171529. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.C.; Fruciano, C.; Marchant, J.; Hildebrand, F.; Forslund, S.; Bork, P.; Engel, P.; Hughes, W.O.H. The gut microbiome is associated with behavioural task in honey bees. Insectes Soc. 2018, 65, 419–429. [Google Scholar] [CrossRef] [Green Version]
- De Souza, D.A.; Kaftanoglu, O.; De Jong, D.; Page, R.E., Jr.; Amdam, G.V.; Wang, Y. Differences in the morphology, physiology and gene expression of honey bee queens and workers reared in vitro versus in situ. Biol. Open 2018, 7, bio036616. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Experimental Design | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Cohort | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Group A | 90 | 60 | 60 | - | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | - |
| Group CA | 20 | 20 | - | - | 20 | 20 | - | - | 20 | - | - | 20 | - | 20 | 20 | - | - |
| Group B | - | 20 | 20 | 20 | - | - | 20 | 20 | 20 | 20 | 20 | 20 | - | 20 | 20 | 20 | 20 |
| Group CB | - | 20 | - | - | - | - | - | 20 | 20 | - | 20 | 20 | - | - | 20 | - | - |
| Method | Organism | PTP3 gBlock® Gene Fragment | Source |
|---|---|---|---|
| qPCR | N. ceranae | 5′_TGAAGCTAAAAAAGAAGAACAACTTGACCAAATAGCTAAAAAGAATGCAGAGACAGAGAAACAACACAGAGAGGTACTTCTCAAAGAACATCAAGATGCTGATGTTATGGCTACAGAAGAAAGACTTGCTAAAAATAATAGAgccaggaaGATTAGTGAGGCAGGAATTAAAGCAGCGCAATCTGTATTGAAAACTGGAGGAACAATAGAAGAAGCAAGAGCAGCTAAGGCGGCAGCTGAAAAAGCTATATTGCAAGAAATTGAGAGTAGAGAAGCGCAAA_3′ | gi|557790804|gb|KC520145.1| |
| Infected Bees (7 Days p.i.) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Cohort | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Group A | n | 30 | 30 | 24 | - | 30 | 30 | 30 | 30 | 21 | 30 | 30 | 30 | 30 | 30 | 30 | 19 * | - |
| qPCR+ | 30 | 29 | 24 | - | 30 | 30 | 30 | 30 | 21 | 30 | 30 | 30 | 30 | 30 | 30 | 19 | - | |
| % | 100 | 97 | 100 | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - | |
| Group B | n | - | 10 | 10 | 10 | - | - | 10 | 10 | 10 | 10 | 10 | 10 | - | 10 | 10 | 10 | 10 |
| qPCR+ | - | 10 | 9 | 10 | - | - | 10 | 10 | 9 | 10 | 9 | 8 | - | 5 | 8 | 1 | 10 | |
| % | - | 100 | 90 | 100 | - | - | 100 | 100 | 90 | 100 | 90 | 80 | - | 50 | 80 | 10 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbieta-Magro, A.; Higes, M.; Meana, A.; Barrios, L.; Martín-Hernández, R. Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae. Insects 2019, 10, 417. https://doi.org/10.3390/insects10120417
Urbieta-Magro A, Higes M, Meana A, Barrios L, Martín-Hernández R. Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae. Insects. 2019; 10(12):417. https://doi.org/10.3390/insects10120417
Chicago/Turabian StyleUrbieta-Magro, Almudena, Mariano Higes, Aránzazu Meana, Laura Barrios, and Raquel Martín-Hernández. 2019. "Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae" Insects 10, no. 12: 417. https://doi.org/10.3390/insects10120417
APA StyleUrbieta-Magro, A., Higes, M., Meana, A., Barrios, L., & Martín-Hernández, R. (2019). Age and Method of Inoculation Influence the Infection of Worker Honey Bees (Apis mellifera) by Nosema ceranae. Insects, 10(12), 417. https://doi.org/10.3390/insects10120417