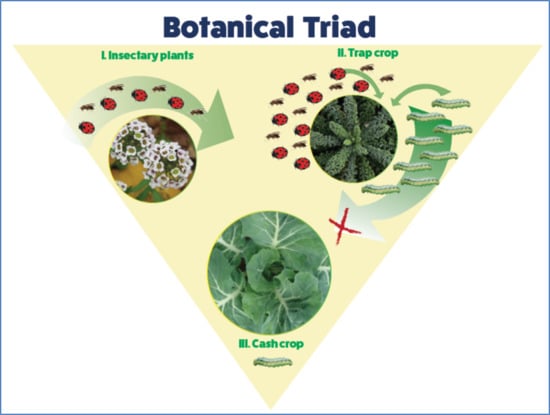
The ‘Botanical Triad’: The Presence of Insectary Plants Enhances Natural Enemy Abundance on Trap Crop Plants in an Organic Cabbage Agro-Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Trap Crops, Insectary Plants, and Cabbage Cash Crop
2.3. Arthropod Sampling
2.4. Statistical Analysis
3. Results
3.1. Herbivore Abundance on Trap Crops
3.2. Natural Enemy Abundance on Trap Crops and Influence of Insectary Plants
3.3. Attractiveness of Insectary Plants to Natural Enemies
3.4. Herbivore Abundance in the Cabbage Cash Crop
3.5. Natural Enemy Abundance in the Cabbage Cash Crop
3.6. Cabbage Cash Crop Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parolin, P.; Bresch, C.; Poncet, C.; Desneux, N. Functional characteristics of secondary plants for increased pest management. Int. J. Pest Manag. 2012, 58, 369–377. [Google Scholar] [CrossRef]
- Parker, J.E.; Crowder, D.W.; Eigenbrode, S.D.; Snyder, W.E. Trap crop diversity enhances crop yield. Agric. Ecosyst. Environ. 2016, 232, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhu, P.Y.; Gurr, G.M.; Zheng, X.S.; Read, D.M.; Heong, K.L.; Yang, Y.J.; Xu, H.X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.F.; Brainard, D.C.; Szendrei, Z. Floral strips attract beneficial insects but do not enhance yield in cucumber fields. J. Econ. Entomol. 2017, 110, 517–524. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Dewenter, I.S.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity- ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Meehan, T.D.; Werling, B.P.; Landis, D.A.; Gratton, C. Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc. Natl. Acad. Sci. USA 2011, 108, 11500–11505. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. Ser. B 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Pontin, D.R.; Wade, M.R.; Kehrli, P.; Wratten, S.D. Attractiveness of single and multiple species flower patches to beneficial insects in agroecosystems. Ann. Appl. Biol. 2006, 148, 39–47. [Google Scholar] [CrossRef]
- Bommarco, R.; Miranda, F.; Bylund, H.; Björkman, C. Insecticides suppress natural enemies and increase pest damage in cabbage. J. Econ. Entomol. 2011, 104, 782–791. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Senapathi, D.; Coston, D.J.; Mortimer, S.R.; Potts, S.G. The benefits of hedgerows for pollinators and natural enemies depends on hedge quality and landscape context. Agric. Ecosyst. Environ. 2017, 247, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Tavares, J.; Wang, K.-H.; Hooks, C.R. An evaluation of insectary plants for management of insect pests in a hydroponic cropping system. Biol. Control 2015, 91, 1–9. [Google Scholar] [CrossRef]
- Lewis, W.J.; van Lenteren, J.C.; Phatak, S.C.; Tumlinson, J.H. A total system approach to sustainable pest management. Proc. Natl. Acad. Sci. USA 1997, 94, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Sivinski, J.; Wahl, D.; Holler, T.; Al Dobai, S.; Sivinski, R. Conserving natural enemies with flowering plants: Estimating floral attractiveness to parasitic Hymenoptera and attraction’s relationship to flower and plant morphology. Biol. Control 2011, 58, 208–214. [Google Scholar] [CrossRef]
- Géneau, C.E.; Wäckers, F.L.; Luka, H.; Daniel, C.; Balmer, O. Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl. Ecol. 2012, 13, 85–93. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. USA 2013, 110, 5534–5539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, K.; Wäckers, F.L.; Valdivia, L.; Larraz, V.; Van Lenteren, J.C. Strategic use of nectar sources to boost biological control. IOBC WPRS Bull. 2003, 29, 165. [Google Scholar]
- Poveda, K.M.; Gomez, M.I.; Martinez, E. Diversification Practices: Their effect on pest regulation and production. Rev. Colomb. Entomol. 2008, 34, 131–144. [Google Scholar]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- Hokkanen, H.M.T. Trap cropping in pest management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review. Insects 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Piñero, J.C.; Manandhar, R. Effects of increased crop diversity using trap crops, flowering plants, and living mulches on vegetable insect pests. Trends Entomol. 2015, 11, 91–109. [Google Scholar]
- Srinivasan, K.; Moorthy, P.N.K. Indian mustard as a trap crop for management of major lepidopterous pests on cabbage. Trop. Pest Manag. 1991, 37, 26–32. [Google Scholar] [CrossRef]
- Luther, G.C.; Valenzuela, H.R.; Defrank, J. Impact of cruciferous trap crops on Lepidopteran pests of cabbage in Hawaii. Environ. Entomol. 1996, 25, 39–47. [Google Scholar] [CrossRef]
- Musser, F.R.; Nault, B.A.; Nyrop, J.P.; Shelton, A.M. Impact of a glossy collard trap crop on diamondback moth adult movement, oviposition, and larval survival. Entomol. Exp. Appl. 2005, 117, 71–81. [Google Scholar] [CrossRef]
- Nafziger, T.D.; Fadamiro, H.Y. Suitability of some farmscaping plants as nectar sources for the parasitoid wasp, Microplitis croceipes (Hymenoptera: Braconidae): Effects on longevity and body nutrients. Biol. Control 2011, 56, 225–229. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.L.; Kaufman, L.V.; Larraz, V.; van Lenteren, J.C. Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol. Control 2009, 50, 299–306. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildflower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control 2015, 91, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Heimpel, G.E. Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 2008, 77, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lavandero, B.; Wratten, S.; Shishehbor, P.; Worner, S. Enhancing the effectiveness of the parasitoid Diadegma semiclausum (Helen): Movement after use of nectar in the field. Biol. Control 2005, 34, 152–158. [Google Scholar] [CrossRef]
- Gillespie, M.; Wratten, S.; Sedcole, R.; Colfer, R. Manipulating floral resources dispersion for hoverflies (Diptera: Syrphidae) in Californian lettuce agro-ecosystem. Biol. Control 2011, 59, 215–220. [Google Scholar] [CrossRef]
- Hogg, B.N.; Bugg, R.L.; Daane, K.M. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 2011, 56, 76–84. [Google Scholar] [CrossRef]
- Shrestha, B. Quantifying the Effects of Insectary Plants on the Abundance of Natural Enemies: Implications for Controlling Insect Pests in an Organic Cabbage Eco-System. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2017. [Google Scholar]
- Triplehorn, C.A.; Johnson, N.F. Borror and Delong’s Introduction to the Study of Insects, 7th ed.; Cengage Learning: Boston, MA, USA, 2004; p. 888. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System). Version 13. 2017. [Google Scholar]
- Mitchell, E.R.; Hu, G.; Johanowicz, D. Management of diamondback moth (Lepidoptera: Plutellidae) in cabbage using collard as a trap crop. HortScience 2000, 5, 875–879. [Google Scholar] [CrossRef]
- Schneider, F. Bionomics and physiology of aphidophagous Syrphidae. Annu. Rev. Entomol. 1969, 14, 103–124. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.J.; France, C.M.; Wratten, S.D.; Frampton, C. Enhancing biological control of leafrollers (Lepidoptera: Tortricidae) by sowing buckwheat (Fagopyrum esculentum) in an orchard. Biocontrol Sci. Technol. 1998, 8, 547–558. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R. Trap crops and insectary plants in the order Brassicales. Ann. Entomol. Soc. Am. 2018, 5, 11–12. [Google Scholar] [CrossRef]
- Vu, Q.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Pasang, J.M.; Almazan, M.L.P.; Ramp, D.; Settele, J.; Horgan, F.G. Enhancing the parasitism of insect herbivores through diversification of habitat in Phillippine rice fields. Paddy Water Environ. 2018, 16, 379–390. [Google Scholar] [CrossRef]
- Gaines, D.N.; Kok, L.T. Cotesia orobenae (Hymenoptera: Braconidae), a gregarious endoparasitoid of Evergestis rimosalis (Lepidoptera: Pyralidae), and hyperparasitoids in Virginia brassica crops. Biol. Control 1995, 5, 573–580. [Google Scholar] [CrossRef]
- Kok, L.T.; Acosta-Martinez, J.A. Differential susceptibility of Cotesia orobenae, a parasitoid of the cross-striped cabbageworm, to commonly used insecticides in Cruciferae. Biocontrol 2001, 46, 419–426. [Google Scholar] [CrossRef]
- Munyaneza, J.; Obrycki, J.J. Development of three populations of Coleomegilla maculata (Coleoptera: Coccinellidae) feeding on eggs of Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1998, 27, 117–122. [Google Scholar] [CrossRef]
- Cavanagh, A.; Hazzard, R.; Adler, L.S.; Boucher, J. Using trap crops for control of Acalymma vittatum (Coleoptera: Chrysomelidae) reduces insecticide use in butternut squash. J. Econ. Entomol. 2009, 102, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wu, K.M.; Wyckhuys, K.A.G.; Guo, Y.Y. Potential of mungbean, Vigna radiatus as a trap crop for managing Apolygus lucorum (Hemiptera: Miridae) on Bt cotton. Crop Prot. 2009, 28, 77–81. [Google Scholar] [CrossRef]
- Javaid, I.; Joshi, J.M. Trap Cropping in Insect Pest Management. J. Sustain. Agric. 1995, 5, 117–136. [Google Scholar] [CrossRef]
- Holden, M.H.; Ellner, S.P.; Lee, D.H.; Nyrop, J.P.; Sanderson, J.P. Designing an effective trap cropping strategy: The effect of attraction, retention and plant spatial distribution. J. Appl. Ecol. 2012, 49, 715–722. [Google Scholar] [CrossRef]
- Hokkanen, H.M.T. Ecostacking: Maximising the benefits of ecosystem services. Arhtropod Plant Interact. 2017, 11, 741–742. [Google Scholar] [CrossRef]







| Natural Enemy Family (# Species Recorded. ND = Not Determined) | Buckwheat | Mixture of Buckwheat and Sweet Alyssum | Sweet Alyssum | Total | % of Total |
|---|---|---|---|---|---|
| Coccinellidae (1) | 81 | 53 | 17 | 151 | 43.5 |
| Syrphidae (ND) | 52 | 52 | 43 | 147 | 42.4 |
| Tachinidae (ND) | 16 | 8 | 5 | 29 | 8.3 |
| Geocoridae (1) | 2 | 1 | 17 | 20 | 5.8 |
| Total | 151 | 114 | 82 | 347 | 100.0 |
| Trap Crop–Insectary Plant Combination | Yield (Grams) Means ± SEM |
|---|---|
| Trap crop present—buckwheat | 1072.50 ± 131.10 |
| Trap crop present—sweet alyssum | 747.25 ± 88.23 |
| Trap crop present—buckwheat and sweet alyssum | 982.50 ± 89.30 |
| Trap crop present—no insectary plants | 995.25 ± 110.71 |
| No trap crop—no insectary plants | 811.09 ± 85.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, B.; Finke, D.L.; Piñero, J.C. The ‘Botanical Triad’: The Presence of Insectary Plants Enhances Natural Enemy Abundance on Trap Crop Plants in an Organic Cabbage Agro-Ecosystem. Insects 2019, 10, 181. https://doi.org/10.3390/insects10060181
Shrestha B, Finke DL, Piñero JC. The ‘Botanical Triad’: The Presence of Insectary Plants Enhances Natural Enemy Abundance on Trap Crop Plants in an Organic Cabbage Agro-Ecosystem. Insects. 2019; 10(6):181. https://doi.org/10.3390/insects10060181
Chicago/Turabian StyleShrestha, Binita, Deborah L. Finke, and Jaime C. Piñero. 2019. "The ‘Botanical Triad’: The Presence of Insectary Plants Enhances Natural Enemy Abundance on Trap Crop Plants in an Organic Cabbage Agro-Ecosystem" Insects 10, no. 6: 181. https://doi.org/10.3390/insects10060181
APA StyleShrestha, B., Finke, D. L., & Piñero, J. C. (2019). The ‘Botanical Triad’: The Presence of Insectary Plants Enhances Natural Enemy Abundance on Trap Crop Plants in an Organic Cabbage Agro-Ecosystem. Insects, 10(6), 181. https://doi.org/10.3390/insects10060181