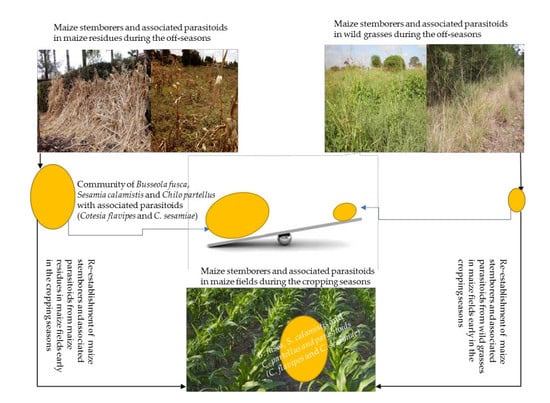
Carry-Over Niches for Lepidopteran Maize Stemborers and Associated Parasitoids during Non-Cropping Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Localities and Sampling Design
2.2. Data Collection
2.2.1. Sampling for the Diversity and Abundance of Lepidopteran Stemborers and Associated Larval and Pupal Parasitoids
In Maize Residues during Non-Cropping Seasons
In Wild Plants during Non-Cropping Seasons
In Maize Plants during Cropping Seasons
2.2.2. Releases of Cotesia flavipes and Cotesia sesamiae in the Studied Farmer’s Fields
2.3. Data Analysis
3. Results
3.1. Diversity and Abundance of Lepidopteran Stemborers and Associated Parasitoids in Maize Residues and Wild Plants during Non-Cropping Seasons
3.2. Relationships in Insect Abundance between Maize Stemborer Species and Their Respective Parasitoids in Either Maize Residues or Wild Plants during Non-Cropping Seasons and in Maize Plants in Cultivated Fields during Subsequent Cropping Seasons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goftishu, M.; Assefa, Y.; Niba, A.; Fininsa, C.; Le Ru, B.P. Diversity and abundance of lepidopteran stem borers and their host plants in Ethiopia. J. Appl. Entomol. 2018, 142, 437–449. [Google Scholar] [CrossRef]
- Le Ru, B.P.; Ong’amo, G.O.; Moyal, P.; Ngala, L.; Musyoka, B.; Abdullah, Z.; Cugala, D.; Defabachew, B.; Haile, T.A.; Matama-Kauma, T.; et al. Diversity of lepidopteran stem borers on monocotyledonous plants in Eastern Africa and the islands of Madagascar and Zanzibar revisited. Bull. Entomol. Res. 2006, 96, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, B.P.; Ong’amo, G.O.; Moyal, P.; Muchugu, E.; Ngala, L.; Musyoka, B.; Abdullah, Z.; Matama-Kauma, T.; Lada, V.Y.; Pallangyo, K.; et al. Geographic distribution and host plant ranges of East African noctuid stem borers. Ann. Soc. Entomol. Fr. 2006, 42, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Maes, K.V.N. Lepidoptera: Introduction. In African Cereal Stem Borers: Economic Importance, Taxonomy, Natural Enemies and Control; Polaszek, A., Ed.; CABI: Wallingford, UK, 1998; pp. 75–78. [Google Scholar]
- Moolman, J.; Van Den Berg, J.; Conlong, D.; Cugala, D.; Siebert, S.; Le Ru, B. Species diversity and distribution of lepidopteran stem borers in South Africa and Mozambique. J. Appl. Entomol. 2014, 138, 52–66. [Google Scholar] [CrossRef]
- Ong’amo, G.O.; Le Gall, P.; Ndemah, R.; Le Ru, P.B. Diversity and host range of lepidopteran stemborer species in Cameroon. Afr. Entomol. 2014, 22, 625–635. [Google Scholar] [CrossRef]
- Ong’amo, G.; Pallangyo, B.; Ali, A.; Njaku, M.; Le Ru, B.P. Diversity and abundance of lepidopteran stem borers and their respective native hosts in different vegetation mosaics in Tanzania. Afr. Entomol. 2018, 26, 50–62. [Google Scholar] [CrossRef]
- Polaszek, A. African Cereal Stem Borers: Economic Importance, Taxonomy, Natural Enemies and Control; CABI: Wallingford, UK, 1998; 530p. [Google Scholar]
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and management of economicaly important lepidopteran cereal stem borers in Africa. Annu. Rev. Entomol. 2002, 47, 701–731. [Google Scholar] [CrossRef] [PubMed]
- De Groote, H.; Doss, C.; Lyimo, S.D.; Mwangi, W.; Alemu, D. Adoption of Maize Technologies in East Africa—What Happened to Africa’s Emerging Maize Revolution? In Proceedings of the FASID Forum V, “Green Revolution in Asia and its Transferability to Africa”, Tokyo, Japan, 8–10 December 2002. [Google Scholar]
- Seshu-Reddy, K.V. Maize and Sorghum: East Africa. In African Cereal Stem Borers: Eco-Nomic Importance, Taxonomy, Natural Enemies and Control; Polaszek, A., Ed.; CABI: Wallingford, UK, 1998; pp. 25–27. [Google Scholar]
- Mailafiya, D.M. Diversity and Ecology Preference of Parasitoids Associated with Lepidopteran Stem Borers in Kenya. Ph.D. Thesis, Kenyatta University, Nairobi City, Kenya, 2009. [Google Scholar]
- Polaszek, A.; Khan, Z.R. Host plants. In African Cereal Stem Borers: Economic Importance, Taxonomy, Natural Enemies and Control; Polaszek, A., Ed.; CABI: Wallingford, UK, 1998; pp. 3–10. [Google Scholar]
- Gebre-Amlak, A. Development of maize stalk borer, Busseola fusca (Fuller) in wild host plants in Ethiopia. J. Appl. Entomol. 1988, 106, 390–395. [Google Scholar] [CrossRef]
- Gebre-Amlak, A. Survival of maize stalk borer, Busseola fusca (Fuller), in crop residues in Ethiopia. Crop Prot. 1988, 7, 183–185. [Google Scholar] [CrossRef]
- Harris, K.M. Lepidopterous stem borers of cereals in Nigeria. Bull. Entomol. Res. 1962, 53, 139–171. [Google Scholar] [CrossRef]
- Ingram, W.R. The Lepidopterous Stalk Borers associated with Gramineae in Uganda. Bull. Entomol. Res. 1958, 49, 367–383. [Google Scholar] [CrossRef]
- Nye, I.W.B. The Insect Pests of Graminaceous Crops in East Africa; Colonial Research Study No. 31; H. M. Stationery Office: London, UK, 1960; 48p. [Google Scholar]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Calatayud, P.-A.; Dupas, S. Species diversity of lepidopteran stem borer parasitoids in cultivated and natural habitats in Kenya. J. Appl. Entomol. 2009, 133, 416–429. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; Okuku, G.; Musyoka, B.; Khadioli, N.; Ong’amo, G.; Le Ru, B. Busseola segeta, a Potential New Pest of Maize in Western Kenya. Entomol. Ornithol. Herpetol. 2014, 3, 132. [Google Scholar] [CrossRef]
- Mushore, K. Assessment of Suitability of Different Populations of Stem Borer Species for the Development of Cotesia flavipes (Hymenoptera: Braconidae) and the Establishment of the Latter in Zimbabwe. Master’s Thesis, University of Zimbabwe, Harare, Zimbabwe, 2005; 97p. [Google Scholar]
- Chinwada, P. Stemborer Parasitism by Cotesia sesamiae and Sturmiopsis parasitica and an Assessment of the Need to Introduce Cotesia flavipes into Zimbabwe. Ph.D. Thesis, Kenyatta University, Nairobi City, Kenya, 2003. [Google Scholar]
- Chinwada, P.; Overholt, W.A. Natural enemies of maize stemborers on the highveld of Zimbabwe. Afr. Entomol. 2001, 9, 67–75. [Google Scholar]
- Midingoyi, S.G.; Affognon, H.D.; Macharia, I.; Ong’amo, G.; Abonyo, E.; Ogola, G.; De Groote, H.; Le Ru, B. Assessing the long-term welfare effects of the biological control of cereal stemborer pests in East and Southern Africa: Evidence from Kenya, Mozambique and Zambia. Agric. Ecosyst. Environ. 2016, 230, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Overholt, W.A.; Ngi-Song, A.J.; Omwega, C.O.; Kimani-Njogu, S.W.; Mbapila, J.; Sallam, M.N.; Ofomata, V. A review of the introduction and establishment of Cotesia flavipes Cameron in East Africa for biological control of cereal stem borers. Insect Sci. Appl. 1997, 17, 79–88. [Google Scholar] [CrossRef]
- Ong’amo, G.; Le Ru, B.; Dupas, S.; Moyal, P.; Muchugu, E.; Calatayud, P.-A.; Silvain, J.-F. The role of wild host plants in the abundance of lepidopteran stem borers along altitudinal gradient in Kenya. Ann. Soc. Entomol. Fr. 2006, 42, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Ong’amo, G.O.; Le Ru, B.P.; Calatayud, P.-A.; Silvain, J.-F. Composition of stem borer communities in selected vegetation mosaics in Kenya. Arthropod-Plant Interact. 2013, 7, 267–275. [Google Scholar] [CrossRef]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Calatayud, P.-A.; Dupas, S. Factors affecting stem borer parasitoid species diversity and parasitism in cultivated and natural habitats. Environ Entomol. 2010, 39, 57–67. [Google Scholar] [CrossRef]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, E.W.; Dupas, S.; Calatayud, P.-A. Parasitism of Lepidopterous stem borers in cultivated and natural habitats. J. Insect Sci. 2011, 11, 1–19. [Google Scholar] [CrossRef]
- Haile, A.; Hofsvang, T. Survey of lepidopterous stem borer pests of sorghum, maize and pearl millet in Eritrea. Crop Prot. 2001, 20, 151–157. [Google Scholar] [CrossRef]
- Kfir, R. Duration of diapause in the stem borers, Busseola fusca and Chilo partellus. Entomol. Exp. Appl. 1991, 61, 265–270. [Google Scholar] [CrossRef]
- Kfir, R.; Van Hamburg, H.; van Vuuren, R. Effect of stubble treatment on the post-diapause emergence of the grain sorghum stalk borer, Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae). Crop Prot. 1989, 8, 289–292. [Google Scholar] [CrossRef]
- Unnithan, G.C.; Reddy, K.V.S. Incidence, diapause and carry over of the cereal stem borers on Rusinga Island, Kenya. Trop. Pest. Manag. 1989, 35, 414–419. [Google Scholar] [CrossRef]
- Usua, E.J. Induction of diapause in the maize stemborer, Busseola fusca. Entomol. Exp. Appl. 1973, 16, 322–328. [Google Scholar] [CrossRef]
- Warui, C.; Kuria, J. Population incidence and control of maize stalk borers Chilo partellus (Swinh.) and C. Orichalcociliellus Strand. and Sesamia calamistis Hmps, Coast Province, Kenya. Insect Sci. Appl. 1983, 4, 11–18. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; ICIPE, Nairobi, Kenya. Personal Obserbation: Smallholder fields and maize stemborers and associated parasitoids statuts observations in the fields in central region of Kenya. 2017. [Google Scholar]
- Ntiri, E.S. Estimating the Impacts of Climate Change on Interactions between Different Lepidopteran Stemborer Species. Ph.D. Thesis, North-West University (Potchefstroom Campus), Potchefstroom, South Africa, 2015. [Google Scholar]
- Sokame, B.M.; Ntiri, S.E.; Ahuya, P.; Baldwyn, T.; Le Ru, B.P.; Kilalo, C.D.; Juma, G.; Calatayud, P.-A. Caterpillar-induced plant volatiles attract conspecific and heterospecific adults for oviposition within a community of lepidopteran stemborers on maize plant. Chemoecology 2019, 29, 89–101. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; pp. 40–44. [Google Scholar]
- Onyango, F.O.; Ochieng’-Odero, J.E.R. Continuous rearing of the maize stem borer Busseola fusca on an artificial diet. Entomol. Exp. Appl. 1994, 73, 139–144. [Google Scholar] [CrossRef]
- Underwood, N. Density dependence in insect performance within individual plants: Induced resistance to Spodoptera exigua in tomato. Oikos 2010, 119, 1993–1999. [Google Scholar] [CrossRef]
- Polaszek, A.; Kimani, S.W. Telenomus species (Hymenopetra: Scelionidae) attacking eggs of pyralid pests (Lepidoptera) in Africa: A review and guide to identification. Bull. Entomol. Res. 1990, 80, 57–71. [Google Scholar] [CrossRef]
- Kenis, M.; du Plessis, H.; Van den Berg, J.; Ba, M.N.; Goergen, G.; Kwardjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafa, G.; et al. Telenomus remus, a Candidate Parasitoid for the Biological Control of Spodoptera frugiperda in Africa, is already Present on the Continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Overholt, W.A.; Ogedah, K.; Lammers, P.M. Distribution and sampling of Chilo partelus (Swinhoe) (Lepidoptera: Pyralydae) in maize and sorghum at Kenya Coast. Bull. Entomol. Res. 1994, 84, 367–378. [Google Scholar] [CrossRef]
- Omwega, C.O.; Muchugu, E.; Overholt, W.A.; Schulthess, F. Release and establishment of Cotesia flavipes Cameron (Hymenoptera: Braconidae) an exotic parasitoid of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) in East and Southern Africa. Ann. Soc. Entomol. Fr. 2006, 42, 511–517. [Google Scholar] [CrossRef]
- Overholt, W.A.; Ochieng, J.O.; Lammers, P.; Ogedah, K. Rearing and field release methods for Cotesia flavipes Cameron (Hymenoptera: Braconidae), a parasitoid of tropical gramineous stem borers. Insect Sci. Appl. 1994, 15, 253–259. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. Available online: https://www.R-project.org/ (accessed on 21 July 2018).
- Di Battista, T.; Fortuna, F.; Maturo, F. BioFTF: An R package for biodiversity assessment with the functional data analysis approach. Ecol. Indic. 2017, 73, 726–732. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement, 1st ed.; Croom Helm Ltd.: Bangor, ME, USA, 1988; 180p. [Google Scholar]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, factoextra; STHDA: UK, 2017; Available online: http://www.sthda.com (accessed on 5 June 2019).
- Ndemah, R.; Schulthess, F.; Le Rü, B.; Bame, I. Lepidopteran cereal stemborers and associated natural enemies on maize and wild grass hosts in Cameroon. J. Appl. Entomol. 2007, 131, 658–668. [Google Scholar] [CrossRef]
- Mailafiya, D.M.; Le Ru, B.P.; Kairu, W.E.; Calatayud., P.-A.; Dupas, S. Geographic distribution, host range and perennation of Cotesia sesamiae and Cotesia flavipes Cameron in cultivated and natural habitats in Kenya. Biol. Control 2010, 54, 1–8. [Google Scholar] [CrossRef]
- Matama-Kauma, T.; Schulthess, F.; Le Ru, B.P.; Mueke, J.; Ogwang, J.A.; Omwega, C.O. Abundance and diversity of lepidopteran stemborers and their parasitoids on selected wild grasses in Uganda. Crop Prot. 2008, 27, 505–513. [Google Scholar] [CrossRef]
- Otieno, N.A.; Le Ru, B.P.; Ong’amo, G.O.; Moyal, P.; Dupas, S.; Calatayud, P.-A.; Silvain, J.-F. Diversity and abundance of wild host plants of lepidopteran stem borers in two agro-ecological zones of Kenya. Int. J. Biodivers. Sci. Manag. 2008, 4, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Otieno, N.A.; Le Ru, B.P.; Ong’amo, G.O.; Dupas, S.; Calatayud, P.-A.; Makobe, M.; Ochora, j.; Silvain, J.-F. Diversity and abundance of wild host plants of lepidopteran stem borers in two different agroecological zones of Kenya. Ann. Soc. Entomol. Fr. 2006, 42, 371–480. [Google Scholar] [CrossRef]
- Ong’amo, G.; Le Ru, B.P.; Dupas, S.; Moyal, P.; Calatayud, P.-A.; Silvain, J.-F. Distribution, pest status and agro-climatic preferences of lepidopteran stem borers of maize in Kenya. Ann. Soc. Entomol. Fr. 2006, 42, 171–177. [Google Scholar] [CrossRef]
- Stewart, A.J.A.; Bantock, T.M.; Beckmann, B.C.; Botham, M.S.; Hubble, D.; Roy, D.B. The role of ecological interactions in determining species ranges and range changes. Biol. J. Linn. Soc. 2015, 115, 647–663. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.; Chapin, F.; Ewel, J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Diniz, S.; Prado, P.I.; Lewinsohn, T.M. Species richness in natural and disturbed habitats: Asteraceae and flower-head insects (Tephritidae: Diptera). Neotrop. Entomol. 2010, 39, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Juma, G.; Ahuya, P.O.; Ong’amo, G.; Le Ru, B.P.; Magoma, G.; Silvain, J.-F.; Calatayud, P.A. Influence of plant silicon in Busseola fusca (Lepidoptera: Noctuidae) larvae—Poaceae interactions. Bull. Entomol. Res. 2015, 105, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Shanower, T.G.; Schulthess, F.; Bosque-Perez, N. The Effect of Larval Diet on the Growth and Development of Sesamia-Calamistis Hampson (Lepidoptera, Noctuidae) and Eldana-Saccharina Walker (Lepidoptera, Pyralidae). Insect Sci. Appl. 1993, 14, 681–685. [Google Scholar] [CrossRef]
- Sétamou, M.; Jiang, N.; Schulthess, F. Effect of the host plant on the survivorship of parasitized Chilo partellus Swinhoe (Lepidoptera: Crambidae) larvae and performance of its larval parasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae). Biol. Control. 2005, 32, 183190. [Google Scholar] [CrossRef]
- Ofomata, V.C.; Overholt, W.A.; Lux, S.A.; Van Huis, A.; Egwuatu, A.R.I. Comparative studies on the fecundity, egg survival, larval feeding, and development of Chilo partellus and Chilo orichalcociliellus (Lepidoptera: Crambidae) on five Grasses. Ann. Entomol. Soc. Am. 2000, 93, 492–499. [Google Scholar] [CrossRef]
- Bonhof, M.J.; Overholt, W.A.; Van Huis, A.; Polaszek, A. Natural Enemies of Cereal Stemborers in East Africa: A Review. Int. J. Trop. Insect Sci. 1997, 17, 19–35. [Google Scholar] [CrossRef]
- Juma, G.; Thiongo, M.; Dutaur, L.; Rharrabe, K.; Le Ru, B.; Magoma, G.; Silvain, J.-F.; Calatayud, P.-A. Two sugar isomers influence host plant acceptance by a cereal caterpillar pest. Bull. Entomol. Res. 2013, 103, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T. Factors inducing and terminating larval diapause in a stem borer, Busseola fusca in Western Kenya. Jpn. Agric. Res. Q. 1991, 25, 47–54. [Google Scholar]
- Ong’amo, G.; Le Ru, B.P.; Moyal, P.; Calatayud, P.-A.; Le Gall, P.; Ogol, C.; Kokwaro, E.D.; Capdevielle-Dualac, C.; Silvain, J.-F. Host plant diversity of Sesamia calamistis: Cytochrome b gene sequences reveal local genetic differentiation. Entomol. Exp. Appl. 2008, 128, 154–161. [Google Scholar] [CrossRef]
- Ndjomatchoua, F.T.; Tonnang, H.E.Z.; Plantamp, C.; Campagne, P.; Tchawoua, C.; Le Ru, B.P. Spatial and temporal spread of maize stem borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae) damage in smallholder farms. Agric. Ecosyst. Environ. 2016, 235, 105–118. [Google Scholar] [CrossRef]
- Gebre-Amlak, A. Phenology and Fecundity of Maize Stalk Borer Busseola fusca (Fuller) in Awassa, Southern Ethiopia. Insect Sci. Appl. 1989, 10, 131–137. [Google Scholar] [CrossRef]
- Kageyama, D.; Hoshizaki, S.; Ishikawa, Y. Female-biased sex ratio in the Asian corn borer, Ostrinia furnacalis: Evidence for the occurrence of feminizing bacteria in an insect. Heredity 1998, 81, 311–316. [Google Scholar] [CrossRef]
- Gempe, T.; Hasselmann, M.; Schiøtt, M.; Hause, G.; Otte, M.; Beye, M. Sex determination in honeybees: Two separate mechanisms induce and maintain the female pathway. PLoS Biol. 2009, 7, e1000222. [Google Scholar] [CrossRef]
- Gempe, T.; Beye, M. Function and evolution of sex determination mechanisms, genes and pathways in insects. BioEssays 2010, 33, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Hasselmann, M.; Gempe, T.; Schiøtt, M.; Nunes-Silva, C.G.; Otte, M.; Beye, M. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 2008, 454, 519–522. [Google Scholar] [CrossRef]
- Hediger, M.; Henggeler, C.; Meier, N.; Perez, R.; Saccone, G.; Bopp, D. Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics 2010, 184, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M. Sex determination in the Teleost Medaka, Oryzias latipes. Annu. Rev. Genet. 2005, 39, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhou, G.; Overholt, W.A.; Muchugu, E.; Schulthess, F. The temporal correlation and spatial synchrony in the stemborer and parasitoid system of Coast Kenya with climate effects. Ann. Soc. Entomol. Fr. 2013, 42, 381–387. [Google Scholar] [CrossRef]
- Overholt, W.; Ngi-Song, A.; Mbapila, J.; Lammers, P.; Kioko, E. Ecological considerations of the introduction of Cotesia flavipes Cameron (Hymenoptera: Braconidae) for biological control of Chilo partellus (Lepidoptera: Pyralidae) in Africa. Biocontrol News Inf. 1994, 15, 19N–24N. [Google Scholar]
- Zhou, G.; Baumgärtner, J.; Overholt, W.A. Impact assessment of an exotic parasitoid on stemborer (Lepidoptera) population dynamics in Kenya. Ecol Appl. 2001, 11, 1554–1562. [Google Scholar] [CrossRef]





| Localities | Sampling/Release Sites | Number of Surveys in Each Habitat | Parasitoid Species Released | No. Adults | No. Cocoon Masses | No. Parasitized Larvae | ||
|---|---|---|---|---|---|---|---|---|
| Maize Residues | Wild Plants | Maize Plants | ||||||
| 2017 | ||||||||
| Makutano | Field 1 | 6 | 6 | 4 | Cotesia flavipes | 500 | 20 | 8 |
| Field 2 | 6 | 6 | 4 | 400 | 20 | 28 | ||
| Field 3 | 6 | 6 | 4 | 400 | - | 10 | ||
| Total | 18 | 18 | 12 | Cotesia flavipes | 1100 | 40 | 46 | |
| Murang’a | Field 1 | 6 | 6 | 4 | Cotesia sesamiae | 300 | 100 | 50 |
| Field 2 | 6 | 6 | 4 | 300 | 30 | 14 | ||
| Field 3 | 6 | 6 | 4 | 400 | 3 | - | ||
| Total | 18 | 18 | 12 | Cotesia sesamiae | 1000 | 133 | 64 | |
| 2018 | ||||||||
| Makutano | Field 1 | 4 | 4 | 4 | Cotesia flavipes | 400 | 40 | - |
| Field 2 | 4 | 4 | 4 | 200 | 20 | - | ||
| Field 3 | 4 | 4 | 4 | 200 | 20 | - | ||
| Total | 12 | 12 | 12 | Cotesia flavipes | 800 | 80 | - | |
| Murang’a | Field 1 | 4 | 4 | 4 | Cotesia sesamiae | 100 | 20 | - |
| Field 2 | 4 | 4 | 4 | 100 | 10 | - | ||
| Field 3 | 4 | 4 | 4 | 50 | 5 | - | ||
| Total | 12 | 12 | 12 | Cotesia sesamiae | 250 | 35 | - | |
| Habitats | Total Number | Stemborer Species Composition | |||
|---|---|---|---|---|---|
| Bf | Sc | Cp | Os | ||
| Makutano | |||||
| Maize residues | 290 | - | 202 (6.73 ± 2.16) | 88 (2.93 ± 0.89) | - |
| Wild plants | 99 | - | 20 (0.67 ± 0.49) | 15 (0.50 ± 0.32) | 61 (2.03 ± 1.50) |
| Sorghum arundinaceum * | 25 | - | 4 | 8 | 9 Csp, 4 Mni |
| Pennisetum purpureum * | 39 | - | 3 | 7 | 29 Csp |
| Megathyrsus maximum * | 22 | - | 7 | - | 15 Mn |
| Cyperus sp. † | 13 | - | 6 | - | 7 Mn |
| Murang’a | |||||
| Maize residues | 363 | 226 (7.53 ± 2.87) | 137 (4.57 ± 1.37) | - | - |
| Wild plants | 33 | 2 (0.06 ± 0.03) | 11 (0.36 ± 0.28) | - | 20 (0.67 ± 0.37) |
| Pennisetum purpureum * | 14 | 9 | - | 5 Sn | |
| Megathyrsus maximum * | 6 | 2 | 1 | - | 3 Mn |
| Cynodon dactylon * | 13 | - | 1 | - | 7 Ssp, 5 Mn |
| Habitats | Makutano | Murang’a | ||||
|---|---|---|---|---|---|---|
| Richness | Shannon | Simpson | Richness | Shannon | Simpson | |
| Maize residues | 2 | 0.627768 | 0.4227348 | 2 | 0.6760856 | 0.4699436 |
| Wild plants | 5 | 1.503721 | 0.7378839 | 5 | 1.5600483 | 0.7584940 |
| Parasitoid Species | Stem Borer Species | Stem Borer Stages | Wild Plants | Total Number of Parasitized Hosts | Makutano | Murang’a | ||
|---|---|---|---|---|---|---|---|---|
| Maize Residues | Wild Plants | Maize Residues | Wild Plants | |||||
| Hymenoptera: Braconidae | ||||||||
| Cotesia flavipes | Cp, Sc | larva | Sa | 9 | 8 | 1 | - | - |
| Cotesia sesamiae | Bf, Sc | larva | - | 12 | - | - | 12 | - |
| Hymenoptera: Ichneumonidae | ||||||||
| Syzectus sp. | - | pupa | Sa, Pm | 3 | - | 3 | - | - |
| Hymenoptera: Eulophidae | ||||||||
| Pediobius furvus | Cp, Sc | pupa | Sa | 4 | 1 | 1 | - | 2 |
| Diptera: Tachinidae | ||||||||
| Siphona (Meigen) sp. | Bf, Mn | larva | Pm | 3 | - | - | - | 3 |
| Habitats | Makutano | Murang’a | ||||
|---|---|---|---|---|---|---|
| Richness | Shannon | Simpson | Richness | Shannon | Simpson | |
| Maize residues | 2 | 0.3638339 | 0.1975309 | 1 | 0.2852830 | 0.1420118 |
| Wild plants | 3 | 0.9824805 | 0.5600000 | 2 | 0.6861506 | 0.4800000 |
| Localities | Total Number | Stemborer Species Composition | ||
|---|---|---|---|---|
| Busseola. fusca | Sesamia calamistis | Chilo partellus | ||
| Makutano | 515 | - | 230 (44.66) | 285 (55.34) |
| Murang’a | 685 | 503 (73.43) | 182 (26.57) | - |
| Total Number | 1200 | 503 (41.92) | 412 (34.33) | 285 (23.75) |
| Localities | Morista–Horn Index (CmH) | |||
|---|---|---|---|---|
| Lepidopteran Maize Stemborer Species | Associated Larval/Pupal Parasitoid Species | |||
| Maize Plants vs. Maize Residues | Maize Plants vs. Wild Plants | Maize Plants vs. Maize Residues | Maize Plants vs. Wild Plants | |
| Makutano | 0.96 | 0.37 | 0.99 | 0.30 |
| Murang’a | 0.97 | 0.31 | 0.99 | 0.02 |
| Parasitoids | Stemborer Species | Parasitism Rates (%) | Proportion Test | ||||
|---|---|---|---|---|---|---|---|
| Maize Plants | Maize Residues | Wild Plants | χ2 | df | p | ||
| Cotesia flavipes (In Makutano) | Sesamia calamistis | 3.48 a | 5.68 a | 5.00 a | 0.8 | 2 | 0.7 |
| Chilo partellus | 2.11 a | 1.49 a | 0.00 a | 0.5 | 2 | 0.8 | |
| Cotesia sesamia (In Murang’a) | Sesamia calamistis | 2.39 a | 3.10 a | 0.00 a | 0.4 | 2 | 0.8 |
| Busseola fusca | 3.85 a | 3.65 a | 0.00 a | 0.4 | 2 | 0.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokame, B.M.; Rebaudo, F.; Musyoka, B.; Obonyo, J.; Mailafiya, D.M.; Le Ru, B.P.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Carry-Over Niches for Lepidopteran Maize Stemborers and Associated Parasitoids during Non-Cropping Season. Insects 2019, 10, 191. https://doi.org/10.3390/insects10070191
Sokame BM, Rebaudo F, Musyoka B, Obonyo J, Mailafiya DM, Le Ru BP, Kilalo DC, Juma G, Calatayud P-A. Carry-Over Niches for Lepidopteran Maize Stemborers and Associated Parasitoids during Non-Cropping Season. Insects. 2019; 10(7):191. https://doi.org/10.3390/insects10070191
Chicago/Turabian StyleSokame, Bonoukpoè Mawuko, François Rebaudo, Boaz Musyoka, Julius Obonyo, Duna Madu Mailafiya, Bruno Pierre Le Ru, Dora Chao Kilalo, Gerald Juma, and Paul-André Calatayud. 2019. "Carry-Over Niches for Lepidopteran Maize Stemborers and Associated Parasitoids during Non-Cropping Season" Insects 10, no. 7: 191. https://doi.org/10.3390/insects10070191
APA StyleSokame, B. M., Rebaudo, F., Musyoka, B., Obonyo, J., Mailafiya, D. M., Le Ru, B. P., Kilalo, D. C., Juma, G., & Calatayud, P. -A. (2019). Carry-Over Niches for Lepidopteran Maize Stemborers and Associated Parasitoids during Non-Cropping Season. Insects, 10(7), 191. https://doi.org/10.3390/insects10070191