The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
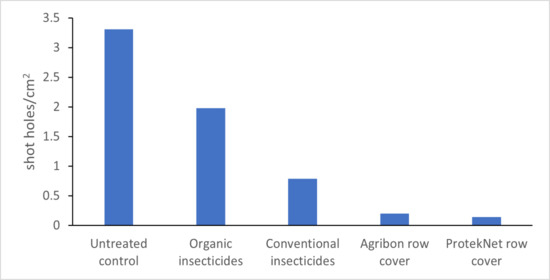
3.1. Spring Trial
3.1.1. Arugula
3.1.2. Mizuna Mustard
3.2. Fall Trial
3.2.1. Arugula
3.2.2. Mizuna Mustard
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 2004 U.S. Farm Bill, s. Specialty Crops Competitiveness Act Section 3. 108th United States Congress. Available online: https://www.congress.gov/bill/108th-congress/house-bill/3242 (accessed on 12 November 2019).
- Penney, U.; Prior, C. Exploring the urban consumer’s perception of local food. Int. J. Retail Distrib. Manag. 2014, 42, 580–594. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Woods, T.; Ernst, M.; Tropp, D. Community Supported Agriculture–New Models for Changing Markets; U.S. Department of Agriculture, Agricultural Marketing Service: Washington, DC, USA, 2017.
- AMS USDA. National Count of Farmer’s Market Directory Listings; U.S. Department of Agriculture, Agricultural Marketing Service: Washington, DC, USA, 2019.
- Hu, W.; Batte, M.T.; Woods, T.; Ernst, S. Consumer preferences for local production and other value-added label claims for a processed food product. Eur. Rev. Agric. Econ. 2012, 39, 489–510. [Google Scholar] [CrossRef]
- Meas, T.; Hu, W.; Batte, M.T.; Woods, T.A.; Ernst, S. Substitutes or complements? Consumer preference for local and organic food attributes. Am. J. Agric. Econ. 2015, 97, 1044–1071. [Google Scholar] [CrossRef]
- Connolly, C.; Klaiber, H.A. Does organic command a premium when the food is already local? Am. J. Agric. Econ. 2014, 96, 1102–1116. [Google Scholar]
- Organic Trade Association. US Organic Industry 2019 Survey. Available online: https://ota.com/what-ota-does/market-analysis/organic-industry-survey/organic-industrysurvey (accessed on 14 November 2019).
- Wolff, B.; Nang, S.L. 3-Year Average Weekly Prices at Kentucky Farmers Markets: 2016–2018; CCD-FS-12; Center for Crop Diversification: Frankfort, KY, USA, 2019. [Google Scholar]
- Knodel, J.J. Flea beetles (Phyllotreta spp.) and their management. In Itegrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops; CAB International: Wallingford, UK, 2017; pp. 1–12. [Google Scholar]
- Tahvanainen, J. The relationship between flea beetles and their cruciferous host plants: The role of plant and habitat characteristics. Oikos 1983, 40, 433–437. [Google Scholar] [CrossRef]
- Hahn, J.W.-B.; Wold-Burkness, S.; Hutchison, W.D.; Hines, R. Flea Beetles; University of Minnesota Extension: Minneapolis, MN, USA, 2018. [Google Scholar]
- Andersen, C.; Hazzard, R.; VanDriesche, R.; Mangan, F. Alternative management tactics for control of Phyllotreta cruciferae and Phyllotreta striolata (Coleoptera: Chrysomelidae) on Brassica rapa in Massachusetts. J. Econ. Entomol. 2006, 99, 803–810. [Google Scholar]
- Walgenbach, J.F.; Schoof, S.C. Cabbage Flea Beetle Insecticide Trial, 2016. Arthropod Manag. Tests 2017, 42, 1–4. [Google Scholar] [CrossRef]
- Seaman, A.J.; Lange, H.W. Evaluation of Insecticides Allowed for Organic Production Against Crucifer Flea Beetle, 2016. Arthropod Manag. Tests 2017, 42, tsx126. [Google Scholar] [CrossRef]
- Turnock, W.; Turnbull, S. The development of resistance to insecticides by the crucifer flea beetle, Phyllotreta cruciferae (Goeze). Can. Entomol. 1994, 126, 1369–1375. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Müller, A.; Heimbach, U.; Nauen, R. Target-site resistance to pyrethroid insecticides in German populations of the cabbage stem flea beetle, Psylliodes chrysocephala L.(Coleoptera: Chrysomelidae). Pestic. Biochem. Physiol. 2014, 108, 1–7. [Google Scholar] [CrossRef]
- Batzer, J.C.; Gleason, M.L. Organic Practices for the Production of Butternut Squash; Research and Demonstration Farms Progress Reports; Iowa State University: Ames, IA, USA, 2011; Volume 2010. [Google Scholar]
- Lamb, R.J. Effects of flea beetles, Phyllotreta spp.(Chrysomelidae: Coleoptera), on the survival, growth, seed yield and quality of canola, rape and yellow mustard. Can. Entomol. 1984, 116, 269–280. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Sowing mixtures of Brassica trap crops is recommended to reduce Phyllotreta beetles injury to cabbage. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 297–303. [Google Scholar]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 52–63. [Google Scholar]
- Atanasova, D.; Leather, S. Plant essential oils: The way forward for aphid control? Ann. Appl. Biol. 2018, 173, 175–179. [Google Scholar] [CrossRef]
- Renkema, J.M.; Wright, D.; Buitenhuis, R.; Hallett, R.H. Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Zabka, M.; Vrchotova, N.; Triska, J.; Kazda, J. Selective effects of the extract from Angelica archangelica L. against Harmonia axyridis (Pallas)—An important predator of aphids. Ind. Crops Prod. 2013, 51, 87–92. [Google Scholar] [CrossRef]
- Atanasova, D.; Ganchev, D.; Nenov, N. In vitro screening for insecticidal activity of natural plant protection products against Tropinota (Epicometis) hirta (Poda) (Coleoptera; Cetoniidae). Agric. Sci. 2017, 9, 47–51. [Google Scholar]
- Atanasova, D.; Nenov, N. Effectiveness of Plant Derived Essentials Oils Products towards Some Aphid (Hemiptera: Aphididae) Species. MAYFEB J. Agric. Sci. 2017, 1, 1–5. [Google Scholar]
- Saroukolai, A.T.; Nouri-Ganbalani, G.; Hadian, J.; Rafiee-Dastjerdi, H. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Prot. Sci. 2014, 50, 207–216. [Google Scholar]
- Ayse, U.B.; Kordali, A.; Kesdek, M.; Altinok, M.; Ercisli, Y.K.S. Toxic effects of eight plant essential oils against adults of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Egypt. J. Biol. Pest Control 2016, 26, 439. [Google Scholar]
- Moretti, M.D.; Peana, A.T.; Franceschini, A.; Carta, C. In vivo activity of Salvia officinalis oil against Botrytis cinerea. J. Essent. Oil Res. 1998, 10, 157–160. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 2010, 58, 457–465. [Google Scholar] [CrossRef]
- Rekika, D.; Stewart, K.A.; Boivin, G.; Jenni, S. Row covers reduce insect populations and damage and improve early season crisphead lettuce production. Int. J. Veg. Sci. 2008, 15, 71–82. [Google Scholar] [CrossRef]
- Aziz, F.; Stewart, K.A.; Jenni, S. Early Growth of Muskmelon in Mulched Minitunnels Containing a Thermal Water Tube. I. Carbon Dioxide Concentrations in the Tunnel. J. Am. Soc. Hortic. Sci. 2001, 126, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Moreno, D.A.; Víllora, G.; Hernández, J.; Castilla, N.; Monreal, L.R. Yield and chemical composition of Chinese cabbage in relation to thermal regime as influenced by row covers. J. Am. Soc. Hortic. Sci. 2002, 127, 343–348. [Google Scholar] [CrossRef]
- Adams, R.G.; Ashley, R.A.; Brennan, M.J. Row covers for excluding insect pests from broccoli and summer squash plantings. J. Econ. Entomol. 1990, 83, 948–954. [Google Scholar] [CrossRef]
- Strang, J.; Hartman, J.; Bessin, R.; Jones, T.; Brown, G.; Barnes, T.; Yankey, T.; Snyder, J. The use of fine meshed netting to exclude pests of thornless blackberries and grapes. HortScience 1992, 27, 1169. [Google Scholar] [CrossRef] [Green Version]
- Chouinard, G.; Firlej, A.; Cormier, D. Going beyond sprays and killing agents: Exclusion, sterilization and disruption for insect pest control in pome and stone fruit orchards. Sci. Hortic. 2016, 208, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Skidmore, A.; Wilson, N.; Williams, M.; Bessin, R. The impact of tillage regime and row cover use on insect pests and yield in organic cucurbit production. Renew. Agric. Food Syst. 2019, 34, 338–348. [Google Scholar] [CrossRef]
- Kuesel, R.; Scott Hicks, D.; Archer, K.; Sciligo, A.; Bessin, R.; Gonthier, D. Effects of fine-mesh exclusion netting on pests of blackberry. Insects 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, D.; Veilleux, J.; Firlej, A. Exclusion net to control spotted wing Drosophila in blueberry fields. IOBC-WPRS Bull. 2015, 109, 181–184. [Google Scholar]
- Link, H.L.; Anderson, K.; Schattman, R.; Méndez, V.E. An Investigation of Insect Netting Trellis Systems to Manage Spotted Wing Drosophila for Vermont Blueberry Farms; Environmental studies electronic thesis collection; University of Vermont: Burlington, VT, USA, 2014; Volume 32. [Google Scholar]
- Leach, H.; Van Timmeren, S.; Isaacs, R. Exclusion netting delays and reduces Drosophila suzukii (Diptera: Drosophilidae) infestation in raspberries. J. Econ. Entomol. 2016, 109, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Burkness, E.C.; Hutchison, W.D. Evaluation of high tunnels for management of Drosophila suzukii in fall-bearing red raspberries: Potential for reducing insecticide use. J. Pest Sci. 2016, 89, 815–821. [Google Scholar] [CrossRef]
- Swezey, S.L.; Nieto, D.J.; Bryer, J.A. Control of western tarnished plant bug Lygus hesperus Knight (Hemiptera: Miridae) in California organic strawberries using alfalfa trap crops and tractor-mounted vacuums. Environ. Entomol. 2014, 36, 1457–1465. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Chouinard, G.; Veilleux, J.; Pelletier, F.; Larose, M.; Philion, V.; Cormier, D. Impact of exclusion netting row covers on arthropod presence and crop damage to ‘Honeycrisp’apple trees in North America: A five-year study. Crop Prot. 2017, 98, 248–254. [Google Scholar] [CrossRef]
- Hilje, L.; Costa, H.S.; Stansly, P.A. Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot. 2001, 20, 801–812. [Google Scholar] [CrossRef]
- Karungi, J.; Obua, T.; Kyamanywa, S.; Mortensen, C.N.; Erbaugh, M. Seedling protection and field practices for management of insect vectors and viral diseases of hot pepper (Capsicum chinense Jacq.) in Uganda. Int. J. Pest Manag. 2013, 59, 103–110. [Google Scholar] [CrossRef]
| Spring Trial 2019 | |
| Control | No spray, no row cover |
| Organic insecticide | Rotation of Spinosad and Pyrethrin sprayed once per week 1 |
| Conventional insecticide | Rotation of Pyrethroid and Dinotefuran sprayed once per week 2 |
| Agribon row cover | Spun-bonded polyethylene row cover 3 |
| ProtekNet row cover | 25-gram fine mesh row cover 4 |
| ProtkeNet and rosemary oil | ProtekNet row cover sprayed with rosemary essential oil twice a week 5 |
| ProtkeNet and thyme oil | ProtekNet row cover sprayed with Thyme essential oil twice a week 5 |
| Fall Trial 2019 | |
| Control | No spray, no row cover |
| Organic insecticide | Rotation of Spinosad and Pyrethrin sprayed once per week 1 |
| Conventional insecticide | Rotation of Pyrethroid and Dinotefuran sprayed once per week 2 |
| Agribon row cover | Spun-bonded polyethylene row cover 3 |
| ProtekNet row cover | 25-gram fine mesh row cover 4 |
| Rosemary oil | Rosemary essential oil applied directly onto greens (no row cover) twice a week 6 |
| Neem oil | Neem essential oil applied directly onto greens (no row cover) twice a week 6 |
| Spring 2019 | |
|---|---|
| Treatment | No. Flea Beetles (Sticky Traps) |
| Control | 16.63 (1.24) A |
| Organic insecticide | 13.63 (1.70) A |
| Conventional insecticide | 5.63 (0.99) B |
| Agribon row cover | 3.13 (0.23) B |
| ProtekNet row cover | 3.13 (0.55) B |
| ProtekNet + Rosemary oil | 1.5 (0.31) B |
| ProtekNet + Thyme oil * | N/A (not applicable) |
| Fall 2019 | |
| Control | 47.81 (11.95) A |
| Organic insecticide | 61.5 (15.38) A |
| Conventional insecticide | 26.5 (6.63) AB |
| Agribon row cover | 8.56 (2.14) C |
| ProtekNet row cover | 3.88 (0.97) BC |
| Rosemary oil | 24.63 (6.16) AB |
| Neem oil | 52.81 (14.11) A |
| Spring 2019 | |||||
| Species | Treatment | Number Flea Beetles (Vacuum) | Damage (holes/cm2) | Yield (Pounds/acre) | Bolting (Stems) |
| Arugula | Control | 3.5 (1.89) A | 0.87 (0.09) C | 12,279 (1261) A | 2.50 (1.55) A |
| Organic insect. | 3.5 (0.65) A | 0.66 (0.07) C | 14,006 (1447) A | 2.25 (1.31) A | |
| Conventional insect. | 6 (3.08) A | 0.25 (0.04) B | 12,046 (794 A | 1.75 (0.85) A | |
| Agribon row cover | 1.25 (0.95) A | 0.07 (0.03) A | 15,874 (1634) A | 7.50 (2.36) B | |
| ProtekNet row cover | 1.25 (0.75) A | 0.07 (0.02) AB | 16,948 (1027) A | 3.75 (2.10) AB | |
| ProtekNet + Rosemary | 0.75 (0.25) A | 0.08 (0.02) AB | 13,540 (1447) A | 3.25 (1.11) A | |
| ProtekNet + Thyme * | N/A | N/A | N/A | N/A | |
| Mizuna | Control | 5.25 (2.25) ab | 1.50 (0.12) b | 10,178 (2288) a | 0 |
| Organic ins. | 7.5 (3.12) b | 1.09 (0.11) b | 10,878 (2334) a | 0 | |
| Conventional ins. | 7.5 (2.53) b | 0.25 (0.05) a | 14,847 (2941) ab | 0 | |
| Agribon row cover | 2.25 (0.75) ab | 0.13 (0.02) a | 17,415 (1074) ab | 0 | |
| ProtekNet row cover | 2.25 (1.03) ab | 0.09 (0.02) a | 21,617 (1587) b | 0 | |
| ProtekNet + Rosemary | 1.25 (0.95) a | 0.17 (0.03) a | 16,247 (2101) ab | 0 | |
| ProtekNet + Thyme * | N/A | N/A | N/A | N/A | |
| Fall 2019 | |||||
| Species | Treatment | Number Flea Beetles (Vacuum) | Damage (holes/cm2) | Yield (Pounds/acre) | Bolting (Stems) |
| Arugula | Control | 57.25 (12.85) B | 3.22 (0.24) C | 9011 (560) AB | 0 |
| Organic ins. | 37.25 (11.20) B | 2.01 (0.21) BC | 9571 (840) A | 0 | |
| Conventional ins. | 5.50 (2.25) A | 1.24 (0.15) B | 8544 (794) AB | 0 | |
| Agribon row cover | 1.50 (0.29) A | 0.28 (0.07) A | 11,672 (1213) A | 0 | |
| ProtekNet row cover | 3.25 (0.85) A | 0.17 (0.03) A | 12,372 (1121) A | 0 | |
| Rosemary oil | 29.50 (2.84) AB | 1.41 (0.15) BC | 3968 (327) B | 0 | |
| Neem oil | 47.25 (8.20) B | 2.19 (0.21) BC | 7143 (1307) AB | 0 | |
| Mizuna | Control | 77.00 (12.25) c | 5.12 (0.37) d | 7844 (420) b | 0 |
| Organic ins. | 50.25 (6.34) b | 2.86 (0.36) c | 9104 (607) ab | 0 | |
| Conventional ins. | 7.75 (1.70) a | 1.32 (0.18) b | 9711 (420) ab | 0 | |
| Agribon row cover | 6.75 (1.31) a | 0.27 (0.04) a | 11,905 (514) ab | 0 | |
| ProtekNet row cover | 6.25 (0.63) a | 0.19 (0.02) a | 15,407 (2101) a | 0 | |
| Rosemary oil | 32.75 (3.42) b | 2.99 (0.27) c | 6443 (1214) b | 0 | |
| Neem oil | 74.25 (0.16) c | 3.58 (0.33) cd | 6303 (980) b | 0 | |
| Fall Temperature 2019 | ||
|---|---|---|
| Treatment | Maximum | Minimum |
| Agribon | 109.6 (0.97) B | 76.3 (0.14) B |
| ProtekNet | 110.1 (0.78) B | 75.8 (0.21) AB |
| Control | 103.6 (0.82) A | 75.3 (0.30) A |
| Spring 2019 | |||
| Species | Treatment Effect | F | p |
| Both | No. flea beetles (sticky traps) | 22.2 | <0.001 |
| Arugula | No. flea beetles (vacuum) | 9.9 (H statistic *) | 0.08 |
| Arugula | Damage (holes/cm2) | 27.8 | <0.001 |
| Arugula | Yield (lbs) | 2.6 | 0.07 |
| Arugula | Bolting | 3.8 | 0.02 |
| Mizuna | No. flea beetles (vacuum) | 4.2 | 0.01 |
| Mizuna | Damage (holes/cm2) | 21.9 | <0.001 |
| Mizuna | Yield (lbs) | 4.8 | 0.008 |
| Mizuna | Bolting | - | - |
| Fall 2019 | |||
| Species | Treatment Effect | F | p |
| Both | No. flea beetles (sticky traps) | 2.1 | 0.15 |
| Both | Temperature maximum | 6.8 | 0.02 |
| Both | Temperature minimum | 11.1 | 0.01 |
| Arugula | No. flea beetles (vacuum) | 9.7 | <0.001 |
| Arugula | Damage (holes/cm2) | 26.1 | <0.001 |
| Arugula | Yield (lbs) | 5.2 | 0.002 |
| Arugula | Bolting | - | - |
| Mizuna | No. flea beetles (vacuum) | 31.8 | <0.001 |
| Mizuna | Damage (holes/cm2) | 124.9 | <0.001 |
| Mizuna | Yield (lbs) | 5.4 | 0.002 |
| Mizuna | Bolting | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockman, R.; Kuesel, R.; Archer, K.; O'Hearn, K.; Wilson, N.; Scott, D.; Williams, M.; Bessin, R.; Gonthier, D. The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production. Insects 2020, 11, 714. https://doi.org/10.3390/insects11100714
Brockman R, Kuesel R, Archer K, O'Hearn K, Wilson N, Scott D, Williams M, Bessin R, Gonthier D. The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production. Insects. 2020; 11(10):714. https://doi.org/10.3390/insects11100714
Chicago/Turabian StyleBrockman, Robert, Ryan Kuesel, Kendall Archer, Kyla O'Hearn, Neil Wilson, Delia Scott, Mark Williams, Ricardo Bessin, and David Gonthier. 2020. "The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production" Insects 11, no. 10: 714. https://doi.org/10.3390/insects11100714
APA StyleBrockman, R., Kuesel, R., Archer, K., O'Hearn, K., Wilson, N., Scott, D., Williams, M., Bessin, R., & Gonthier, D. (2020). The Impact of Plant Essential Oils and Fine Mesh Row Covers on Flea Beetle (Chrysomelidae) Management in Brassicaceous Greens Production. Insects, 11(10), 714. https://doi.org/10.3390/insects11100714