Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys)
Abstract
:1. Introduction
2. Material and Methods
2.1. Laboratory Colony
2.2. Developmental Time and Life Table Studies
2.3. Statistical Analysis
3. Results
3.1. Developmental Time, Adult Longevity and Lifespan
3.2. Pre-Oviposition Period, Oviposition Period and Fecundity
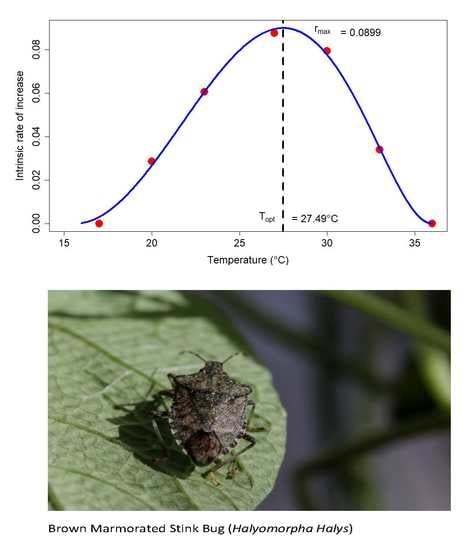
3.3. Life Table Analysis
3.4. Comparison of Development and Survival Rates for the MN versus PA populations of H. halys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, R.C. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press Inc.: New York, NY, USA, 1993; ISBN 0-19-506687-1. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Fu, J.; You, M. Age-stage, two-sex life table and its application in population ecology and integrated pest management. Acta Entomol. Sin. 2019, 62, 255–262. [Google Scholar]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Hogg, D.B. Demographic statistics for the pea aphid (Homoptera: Aphididae) in Wisconsin and a comparison with other populations. Environ. Entomol. 1984, 15, 1173–1181. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Mironidis, G.K. Development, survivorship and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under fluctuating temperatures. Bull. Entomol. Res. 2014, 104, 751–764. [Google Scholar] [CrossRef]
- Musolin, D.L.; Dolgovskaya, M.Y.; Protsenko, V.Y.; Karpun, N.N.; Reznik, S.Y.; Saulich, A.K. Photoperiodic and temperature control of nymphal growth and adult diapause induction in the invasive Caucasian population of the brown marmorated stink bug. Halyomorpha halys. J. Pest Sci. 2019, 92, 621–631. [Google Scholar] [CrossRef]
- Estay, S.A.; Lima, M.; Bozinovic, F. The role of temperature variability on insect performance and population dynamics in a warming world. Oikos 2014, 123, 131–140. [Google Scholar] [CrossRef]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Christopher Bergh, J.; Ames Herbert, D.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Leskey, T.C.; Nielsen, A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriticos, D.J.; Kean, J.M.; Phillips, C.B.; Senay, S.D.; Acosta, H.; Haye, T. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 2017, 90, 1033–1043. [Google Scholar] [CrossRef]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Fogain, R.; Graff, S. First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ont. 2011, 142, 45–48. [Google Scholar]
- Faúndez, E.I.; Rider, D. The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arq. Entomolóxicos 2017, 17, 305–307. [Google Scholar]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Bull. Société Entomol. Suisse 2008, 81, 1–8. [Google Scholar]
- Arnold, K. Halyomorpha halys (Stål, 1855), a stink bug species newly detected among the European fauna (Insecta: Heteroptera, Pentatomidae, Pentatominae, Cappaeini). Mitt. Thuring. Entomol. 2009, e.V.16:10. [Google Scholar]
- Heckmann, R. First evidence of Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Germany. Heteropteron H 2012, 36, 17–18. [Google Scholar]
- Callot, H.; Brua, C. Halyomorpha halys (Stål, 1855), la Punaise diabolique, nouvelle espèce pour la faune de France (Heteroptera Pentatomidae). L’ Entomol. 2013, 69, 69–71. [Google Scholar]
- Maistrello, L.; Dioli, P.; Dutto, M.; Volani, S.; Pasquali, S.; Gilioli, G. Tracking the spread of sneaking aliens by integrating crowdsourcing and spatial modeling: The Italian invasion of Halyomorpha halys. Bioscience 2018, 12, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Milonas, P.G.; Partsinevelos, G.K. First report of brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) in Greece. EPPO Bull. 2014, 44, 183–186. [Google Scholar] [CrossRef]
- Vétek, G.; Papp, V.; Haltrich, A.; Rédei, D. First record of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae), in Hungary, with description of the genitalia of both sexes. Zootaxa 2014, 3780, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macavei, L.I.; Baetan, R.; Oltean, I.; Florian, T.; Varga, M.; Costi, E.; Maistrello, L. First detection of Halyomorpha halys Stål, a new invasive species with a high potential of damage on agricultural crops in Romania. Lucr. Ştiinţifice 2015, 58, 105–108. [Google Scholar]
- Šeat, J. Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) a new invasive species in Serbia. Acta Entomol. Serbica 2015, 20, 167–171. [Google Scholar]
- Murvanidze, M.; Krawczyk, G.; Inasaridze, N.; Dekanoidze, L.; Samsonadze, N.; Macharashvili, M.; Khutsishvili, S.; Shengelaia, S. Preliminary data on the biology of brown marmorated stink bug Halyomorpha halys (Hemiptera, Pentatomidae) in Georgia. Turk. J. Zool. 2018, 42, 617–624. [Google Scholar] [CrossRef]
- Dioli, P.; Leo, P.; Maistrello, L. First records in Spain and Sardinia of the alien species Halyomorpha halys (Stal, 1855), with notes on its distribution in Europe (Hemiptera, Pentatomidae). Rev. Gaditana Entomol. 2016, VII, 539–548. [Google Scholar]
- Gapon, D.A. First records of the brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera, Pentatomidae) in Russia, Abkhazia, and Georgia. Entomol. Rev. 2016, 96, 1086–1088. [Google Scholar] [CrossRef]
- Musolin, D.L.; Konjević, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: History of invasion, range expansion, early stages of establishment, and first records of damage to local crops. Arthropod Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Simov, N. The invasive brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) already in Bulgaria. Ecol. Montenegrina 2016, 9, 51–53. [Google Scholar]
- Hemala, V.; Kment, P. First record of Halyomorpha halys and mass occurrence of Nezara viridula in Slovakia. Plant Prot. Sci. 2017, 53, 247–253. [Google Scholar]
- Esenbekova, P.A. First record of Halyomorpha halys (Stål, 1855) (Heteroptera, Pentatomidae) from Kazakhstan. Eurasian Entomol. J. 2017, 16, 23–24. [Google Scholar]
- Šapina, I.; Jelaska, L.Š. First report of invasive brown marmorated stink bug Halyomorpha halys (Stål, 1855) in Croatia. EPPO Bull. 2018, 48, 138–143. [Google Scholar] [CrossRef]
- Güncan, A.; Gümüş, E. Brown marmorated stink bug, Halyomorpha halys (StåL, 1855) (Hemiptera: Heteroptera, Pentatomidae), a new and important pest in Turkey. Entomol. News 2019, 128, 204–210. [Google Scholar] [CrossRef]
- Tassini, C.; Mifsud, D. The brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) in Malta. EPPO Bull. 2019, 49, 132–136. [Google Scholar] [CrossRef]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stal) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Washingt. 2003, 105, 225–237. [Google Scholar]
- American/Western Fruit Grower. Brown Marmorated Stink Bug Causes $37 Million in Losses to Mid-Atlantic Apple Growers. 14 April 2011. Available online: http://www.growingproduce.com/article/21057/brown-marmorated-stink-bug-causes-37-million-in-losses-to-mid-atlantic-apple-growers (accessed on 17 September 2011).
- Koch, R.L. Detections of the brown marmorated stink bug (Hemiptera: Pentatomidae) in Minnesota. J. Entomol. Sci. 2014, 49, 313–317. [Google Scholar] [CrossRef]
- Cira, T.M.; Venette, R.C.; Aigner, J.; Kuhar, T.; Mullins, D.E.; Gabbert, S.E.; Hutchison, W.D. Cold tolerance of Halyomorpha halys (Hemiptera: Pentatomidae) across geographic and temporal scales. Environ. Entomol. 2016, 45, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Pezzini, D.T.; DiFonzo, C.D.; Finke, D.L.; Hunt, T.E.; Knodel, J.J.; Krupke, C.H.; McCornack, B.; Michel, A.P.; Moon, R.D.; Philips, C.R.; et al. Spatial patterns and sequential sampling plans for estimating densities of stink bugs (Hemiptera: Pentatomidae) in Soybean in the north central region of the United States. J. Econ. Entomol. 2019, 112, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Costi, E.; Haye, T.; Maistrello, L. Biological parameters of the invasive brown marmorated stink bug, Halyomorpha halys, in southern Europe. J. Pest Sci. 2017, 90, 1059–1067. [Google Scholar] [CrossRef]
- Haye, T.; Abdallah, S.; Gariepy, T.; Wyniger, D. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J. Pest Sci. 2014, 87, 407–418. [Google Scholar] [CrossRef]
- Baek, S.; Hwang, A.; Kim, H.; Lee, H.; Lee, J.H. Temperature-dependent development and oviposition models of Halyomorpha halys (Hemiptera: Pentatomidae). J. Asia. Pac. Entomol. 2017, 20, 367–375. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C.; Matadha, D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef]
- Maslen, E.A. The Impact of Temperature on Halyomorpha halys (Hemiptera: Pentatomidae) Life Table Parameters and Feeding Pressure. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2016. [Google Scholar]
- Nielsen, A.L.; Chen, S.; Fleischer, S.J. Coupling developmental physiology, photoperiod, and temperature to model phenology and dynamics of an invasive heteropteran, Halyomorpha halys. Front. Physiol. 2016, 7, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin, R.E.; Nielsen, A.L.; Wiman, N.G.; Lee, D.H.; Fonseca, D.M. Global invasion network of the brown marmorated stink bug, Halyomorpha halys. Sci. Rep. 2017, 7, 9866. [Google Scholar] [CrossRef] [Green Version]
- Iverson, J.M.; Cira, T.M.; Burkness, E.C.; Hutchison, W.D. Cannibalistic oophagy in Halyomorpha halys (Hemiptera: Pentatomidae) laboratory colonies. J. Entomol. Sci. 2016, 51, 122–128. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Agnello, A.M.; Alston, D.G.; Andrews, H.; Beers, E.H.; Christopher Bergh, J.; Bessin, R.; Blaauw, B.R.; Buntin, G.D.; Burkness, E.C.; et al. Season-long monitoring of the brown marmorated stink bug (Hemiptera: Pentatomidae) throughout the United States using commercially available traps and lures. J. Econ. Entomol. 2019, 1–13. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart-exe-B100000.rar (Version 2019.03.12) (accessed on 5 August 2019).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reprodu. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, W.; Sun, Y.; Feng, J. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: London, UK, 1993; ISBN 0412042312. [Google Scholar]
- Amarasekare, P.; Savage, V. A framework for elucidating the temperature dependence of fitness. Am. Nat. 2012, 179, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Li, B.-L.; Ge, F. Intrinsic optimum temperature of the diamondback moth and its ecological meaning. Environ. Entomol. 2012, 41, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Bonato, O.; Lurette, A.; Vidal, C.; Fargues, J. Modelling temperature-dependent bionomics of Bemisia tabaci (Q-biotype). Physiol. Entomol. 2007, 9, 1144–1150. [Google Scholar] [CrossRef]
- Rebaudo, F.; Struelens, Q.; Dangles, O. Modelling temperature-dependent development rate and phenology in arthropods: The devRate package for r. Methods Ecol. Evol. 2018, 9, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Ikemoto, T.; Egami, C.; Sun, Y.; Ge, F. A modified program for estimating the parameters of the SSI model. Environ. Entomol. 2011, 40, 462–469. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 15 July 2019).
- Burnham, K.; Anderson, D. Model Selection and Multi-Model Inference; Springer-Verlag: New York, NY, USA, 2002; ISBN 0387953647. [Google Scholar]
- Koch, R.L.; Rich, W.A. Stink bug (Hemiptera: Heteroptera: Pentatomidae) feeding and phenology on early-maturing soybean in Minnesota. J. Econ. Entomol. 2015, 108, 2335–2343. [Google Scholar] [CrossRef]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche 2012. [Google Scholar] [CrossRef]
- Simmons, A.M.; Yeargan, K.V. Development and survivorship of the green stink bug, Acrosternum hilare (Hemiptera: Pentatomidae) on soybean. Environ. Entomol. 1988, 17, 527–532. [Google Scholar] [CrossRef]
- Da Silva, P.G.; Daane, K.M. Life history parameters of Chinavia hilaris (Hemiptera: Pentatomidae), a stink bug injurious to pistachios in California. J. Econ. Entomol. 2014, 107, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemoto, T. Intrinsic optimum temperature for development of insects and mites. Environ. Entomol. 2005, 34, 1377–1387. [Google Scholar] [CrossRef]
- Martins, J.C.; Picanço, M.C.; Bacci, L.; Guedes, R.N.C.; Santana, P.A.; Ferreira, D.O.; Chediak, M. Life table determination of thermal requirements of the tomato borer Tuta Absol. J. Pest Sci. 2016, 89, 897–908. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J. Temperature and the catalytic activity of enzymes: A fresh understanding. FEBS Lett. 2013, 587, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Dingha, B.N.; Jackai, L.E.N. Laboratory rearing of the brown marmorated stink bug (Hemiptera: Pentatomidae) and the impact of single and combination of food substrates on development and survival. Can. Entomol. 2017, 149, 1–14. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Story, R.N. Adaptive functions of nymphal aggregation in the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatomidae). Environ. Entomol. 1986, 15, 739–749. [Google Scholar] [CrossRef]
- Xu, J.; Fonseca, D.M.; Hamilton, G.C.; Hoelmer, K.A.; Nielsen, A.L. Tracing the origin of US brown marmorated stink bugs, Halyomorpha halys. Biol. Invasions 2014, 16, 153–166. [Google Scholar] [CrossRef]
- Valentin, R.E.; Maslo, B.; Lockwood, J.L.; Pote, J.; Fonseca, D.M. Real-time PCR assay to detect brown marmorated stink bug, Halyomorpha halys (Stål), in environmental DNA. Pest Manag. Sci. 2016, 72, 1854–1861. [Google Scholar] [CrossRef]
- Lee, W.; Guidetti, R.; Cesari, M.; Gariepy, T.D.; Park, Y.-L.; Park, C.-G. Genetic diversity of Halyomorpha halys (Hemiptera, Pentatomidae) in Korea and comparison with COI sequence datasets from East Asia, Europe, and North America. Fla. Entomol. 2018, 101, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Morrison, W.R.; Milonas, P.; Kapantaidaki, D.E.; Cesari, M.; Di Bella, E.; Guidetti, R.; Haye, T.; Maistrello, L.; Moraglio, S.T.; Piemontese, L.; et al. Attraction of Halyomorpha halys (Hemiptera: Pentatomidae) haplotypes in North America and Europe to baited traps. Sci. Rep. 2017, 7, 16941. [Google Scholar] [CrossRef]
- Kapantaidaki, D.E.; Evangelou, V.I.; Morrison, W.R.; Leskey, T.C.; Brodeur, J.; Milonas, P. Halyomorpha halys (Hemiptera: Pentatomidae) genetic diversity in North America and Europe. Insects 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briere, J.F.; Pracros, P.; Le Roux, A.Y.; Pierre, J.S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesari, M.; Maistrello, L.; Piemontese, L.; Bonini, R.; Dioli, P.; Lee, W.; Park, C.G.; Partsinevelos, G.K.; Rebecchi, L.; Guidetti, R. Genetic diversity of the brown marmorated stink bug Halyomorpha halys in the invaded territories of Europe and its patterns of diffusion in Italy. Biol. Invasions 2018, 20, 1073–1092. [Google Scholar] [CrossRef]
- Kaser, J.M.; Akotsen-Mensah, C.; Talamas, E.J.; Nielsen, A.L. First report of Trissolcus japonicus parasitizing Halyomorpha halys in North American Agriculture. Fla. Entomol. 2019, 101, 680–683. [Google Scholar] [CrossRef]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; ISBN 9781107415324. [Google Scholar]
- Venugopal, P.D.; Dively, G.P.; Herbert, A.; Malone, S.; Whalen, J.; Lamp, W.O. Contrasting role of temperature in structuring regional patterns of invasive and native pestilential stink bugs. PLoS ONE 2016, 11, e0150649. [Google Scholar] [CrossRef]





| Temperature (°C) | n | Egg | First Instar | Second Instar | Third Instar | Fourth Instar | Fifth Instar | Pre-adult | Survival (%) |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 59 | 21.01± 0.07 a | 26.57 ± 0.18 a | — | — | — | — | — | 0.00 |
| 17 | 50 | 13.23 ± 0.04 b | 13.20 ± 0.14 b | 24.95 ± 0.80 a | 22.16 ± 1.55 a | 21.43 ± 2.18 a | 19.50 ± 0.66 a | 105.89 ± 4.69 a | 18.00 |
| 20 | 35 | 12.71 ± 0.11 c | 10.90 ± 0.10 c | 18.77 ± 0.37 b | 12.56 ± 0.90 b | 11.15 ± 0.49 b | 16.46 ± 0.45 a | 80.67 ± 1.04 b | 68.00 |
| 23 | 51 | 5.61 ± 0.09 d | 4.95 ± 0.06 d | 11.13 ± 0.29 c | 8.39 ± 0.30 c | 7.90 ± 0.38 c | 11.47 ± 0.42 b,e | 49.07 ± 1.06 c | 82.00 |
| 25 | 51 | 5.60 ± 0.07 d | 5.06 ± 0.10 d | 9.58 ± 0.18 d | 7.07 ± 0.17 c,d | 6.52 ± 0.11 c,d | 9.06 ± 0.08 c | 42.62 ± 0.38 d | 86.00 |
| 27 | 50 | 3.25 ± 0.04 e | 3.80 ± 0.04 e | 7.98 ± 0.15 e | 5.41 ± 0.11 d | 5.38 ± 0.11 d | 7.46 ± 0.12 d | 33.21 ± 0.36 e | 96.00 |
| 30 | 50 | 3.00 ± 0.00 e | 3.02 ± 0.07 f | 6.60 ± 0.19 f | 5.41 ± 0.14 d | 5.31 ± 0.13 d | 8.01 ± 0.25 c,d | 30.95 ± 0.45 e | 80.00 |
| 33 | 56 | 3.76 ± 0.10 f | 3.25 ± 0.04 f | 5.43 ± 0.09 g | 6.04 ± 0.22 d,e | 6.74 ± 0.53 c,d | 8.20 ± 0.25 c,d | 31.20 ± 0.56 e | 10.00 |
| 36 | 133 | 3.14 ± 0.03 e | 3.27 ± 0.05 f | 6.63 ± 0.12 f | 7.55 ± 0.31 c,e | 5.83 ± 0.28 c,d | 8.75 ± 0.18 c,d,e | 32.17 ± 0.63 e | 2.00 |
| Temperature (°C) | Sample Size (Mating Pairs) | Female Adult Longevity (days) | Male Adult Longevity (days) | Pre-oviposition Period (days) | Oviposition Period (days) | Fecundity (eggs/female life span) |
|---|---|---|---|---|---|---|
| 17 * | 4 | 227.88 ± 8.27 a | 211.4 ± 8.89 a | - | - | - |
| 20 | 10 | 181.5 ± 15.39 b | 180.75 ± 6.69 b | 32.67± 1.37 a | 70.13 ± 12.33 a | 199.8 ± 49.57 a,c |
| 23 | 12 | 130.08 ± 7.62 c | 119.12 ± 8.7 c | 14.67 ± 1.09 b | 59.25 ± 5.33 a | 308.67 ± 30.58 a |
| 27 | 13 | 85.96 ± 9.72 d | 61.20 ± 4.69 d | 11.54 ± 0.76 c | 38.71 ± 8.37 a,b | 278.92 ± 57.05 a |
| 30 | 19 | 84.68 ± 6.00 d | 83.62 ± 5.35 e | 10.84 ± 0.74 c | 26.25 ± 4.70 b | 152.84 ± 15.98 b,c |
| 33 | 3 | 90.67 ± 13.15 d | 89.83 ± 9.81 c,d,e | 11.00 ± 0.00 c | 24.17 ± 11.46 a,b | 121.00 ± 33.08 b,c |
| Temperature (°C) | n | T | R0 | λ | GRR | la | |
|---|---|---|---|---|---|---|---|
| 20 | 29 | 145.60 ± 5.89 a | 68.82 ± 23.96 a,b | 0.0286 ± 0.00 a | 1.029 ± 0.00 a | 229.4 ± 84.03 a,b,c,d | 0.6896 ± 0.09 a |
| 23 | 29 | 79.60 ± 1.95 b | 127.73 ± 30.77 b | 0.0606 ± 0.00 b | 1.062 ± 0.00 b | 189.24 ± 40.02 a | 0.8273 ± 0.07 b,c |
| 27 | 24 | 56.83 ± 1.93 c | 151.06 ± 41.16 b | 0.0876 ± 0.00 c | 1.092 ± 0.00 c | 401.65 ± 55.57 b | 0.9582 ± 0.04 b |
| 30 | 50 | 50.94 ± 1.55 d | 58.12 ± 12.02 a | 0.0794 ± 0.00 c | 1.083 ± 0.01 c | 98.42 ± 19.98 c | 0.7999 ± 0.06 c |
| 33 | 56 | 51.20 ± 3.74 d | 6.80 ± 3.75 c | 0.0339 ± 0.01 a | 1.035 ± 0.01 a | 66.81 ± 31.86 d | 0.1096 ± 0.04 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindan, B.N.; Hutchison, W.D. Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects 2020, 11, 108. https://doi.org/10.3390/insects11020108
Govindan BN, Hutchison WD. Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects. 2020; 11(2):108. https://doi.org/10.3390/insects11020108
Chicago/Turabian StyleGovindan, Byju N., and William D. Hutchison. 2020. "Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys)" Insects 11, no. 2: 108. https://doi.org/10.3390/insects11020108
APA StyleGovindan, B. N., & Hutchison, W. D. (2020). Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects, 11(2), 108. https://doi.org/10.3390/insects11020108