Floral Species Richness Correlates with Changes in the Nutritional Quality of Larval Diets in a Stingless Bee
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Study Species
2.3. Sampling of Larval Provisions
2.4. Nutritional Analyses
2.4.1. Amino Acid Analysis
2.4.2. Fatty Acid Analysis
2.5. Metabarcoding
2.6. Statistical Analysis
2.6.1. Effects of Surrounding Plant Species Richness and Habitat on Nutrient Contents and Ratios in Larval Nutrition
2.6.2. Effects of Surrounding Plant Species Richness and Habitat on Nutrient Composition in Larval Nutrition
3. Results
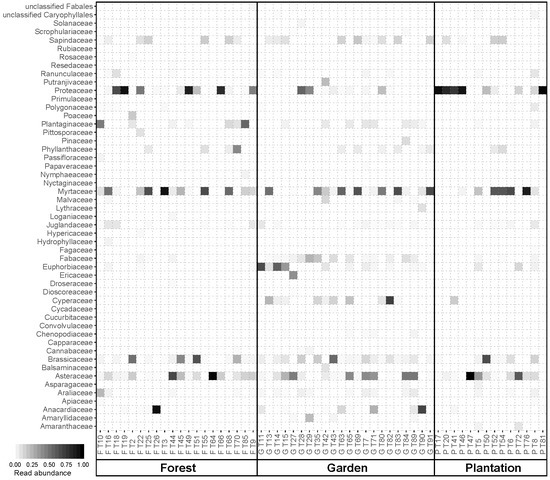
3.1. Floral Composition and Diversity
3.2. Nutrient Contents and Ratios
3.3. Nutrient Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolson, S.W. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2017, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.; Fluri, P.; Imdorf, A. Pollen nutrition and colony development in honey bees: Part i. Bee World 2005, 86, 3–10. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; John Hopkins University Press: Baltimore & London, UK, 2007; p. 953. [Google Scholar]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Waser, N.M.; Ollerton, J. Plant-Pollinator Interactions: From Specialization to Generalization; The University of Chicago Press: Chicago, LI, USA, 2006; p. 488. [Google Scholar]
- Wcislo, W.T.; Cane, J.H. Floral resource utilization by solitary bees (hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annu. Rev. Entomol. 1996, 41, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Darvill, B. Niche overlap and diet breadth in bumblebees; are rare species more specialized in their choice of flowers? Apidologie 2004, 35, 55–63. [Google Scholar] [CrossRef]
- Kämper, W.; Werner, P.K.; Hilpert, A.; Westphal, C.; Blüthgen, N.; Eltz, T.; Leonhardt, S.D. How landscape, pollen intake and pollen quality interact to affect colony growth in bombus terrestris? Landsc. Ecol. 2016, 31, 2245. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional composition of honey bee food stores vary with floral composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Levin, M.D.; Haydak, M.H. Comparative value of different pollens in the nutrition of osmia lignaria. Bee World 1957, 38, 221–226. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhardt, M.; Haider, M.; Dorn, S.; Müller, A. Pollen mixing in pollen generalist solitary bees: A possible strategy to complement or mitigate unfavourable pollen properties? J. Anim. Ecol. 2014, 83, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Moerman, R.; Vanderplanck, M.; Fournier, D.; Jacquemart, A.-L.; Michez, D. Pollen nutrients better explain bumblebee colony development than pollen diversity. Insect Conserv. Divers. 2017, 10, 171–179. [Google Scholar] [CrossRef]
- MacDougall, A.S.; McCann, K.S.; Gellner, G.; Turkington, R. Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse. Nature 2013, 494, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geist, H.J.; Lambin, E.F. Proximate causes and underlying driving forces of tropical deforestation. Bioscience 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vazquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Roulston, T.H.; Goodell, K. The role of resources and risks in regulating wild bee populations. Annu. Rev. Entomol. 2011, 56, 293–312. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; The Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, C.N.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Land use intensity in grasslands: Changes in biodiversity, species composition and specialisation in flower visitor networks. Basic Appl. Ecol. 2011, 12, 292–299. [Google Scholar] [CrossRef]
- Duffy, J.E. Biodiversity and ecosystem function: The consumer connection. Oikos 2001, 99, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Duffy, J.E.; Carinale, B.J.; France, K.E.; McIntyre, P.B.; Thebault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant diversity and productivity experiments in european grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol. Lett. 2005, 8, 696–703. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Naug, D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014, 4, 4195–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaudo, A.D.; Farrell, L.M.; Patch, H.M.; Grozinger, C.M.; Tooker, J.F. Consistent pollen nutritional intake drives bumble bee (Bombus impatiens) colony growth and reproduction across different habitats. Ecol. Evol. 2018, 8, 5765–5776. [Google Scholar] [CrossRef] [PubMed]
- Hartfelder, K.; Engels, W. The composition of larval food in stingless bees: Evaluating nutritional balance by chemosystematic methods. Insect. Soc. 1989, 36, 1–14. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.M.; Keller, A.; Heard, T.A.; Jeffers, B.; Drescher, N.; Blüthgen, N.; Leonhardt, S.D. Generalist social bees maximize diversity intake in plant species-rich and resource-abundant environments. Ecosphere 2017, 8, e01758. [Google Scholar] [CrossRef] [Green Version]
- Vaudo, A.D.; Patch, H.M.; Mortensen, D.A.; Tooker, J.F.; Grozinger, C.M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl. Acad. Sci. USA 2016, 113, E4035–E4042. [Google Scholar] [CrossRef] [Green Version]
- Vaudo, A.D.; Stabler, D.; Patch, H.M.; Tooker, J.F.; Grozinger, C.M.; Wright, G.A. Bumble bees regulate their intake of the essential protein and lipid pollen macronutrients. J. Exp. Biol. 2016, 219. [Google Scholar] [CrossRef] [Green Version]
- Hendriksma, H.P.; Shafir, S. Honey bee foragers balance colony nutritional deficiencies. Behav. Ecol. Sociobiol. 2016, 70, 509–517. [Google Scholar] [CrossRef]
- Zarchin, S.; Dag, A.; Salomon, M.; Hendriksma, H.P.; Shafir, S. Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav. Ecol. Sociobiol. 2017, 71, 172. [Google Scholar] [CrossRef]
- Needler, V.J.; Niehus, R.E.; Wilson, B.A.; McDonald, W.J.F.; Ford, A.J.; Accad, A. The Vegetation of Queensland. Descriptions of Broad Vegetation Groups; Queensland Herbarium, Department of Environment and Science: Brisbane, Australia, 2019; Version 4.0. [Google Scholar]
- Bostock, P.D.; Holland, A.E. Introduction to the Census of the Queensland Flor; Queensland Department of Environment and Science: Brisbane, Australia, 2018. [Google Scholar]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Klein, A.-M.; Leonhardt, S.D. Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 2016, 6, 1304–1316. [Google Scholar] [CrossRef] [Green Version]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.M.; Leonhardt, S.D. Social bees are fitter in more biodiverse environments. Sci. Rep. 2018, 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Heard, T.A.; Gloag, A.R.; Beekman, M. Flight range of the Australian stingless bee, Tetragonula carbonaria (Hymenoptera, Apidae). Austral Entomol. 2016, in press. [Google Scholar] [CrossRef]
- Dollin, A.E.; Dollin, L.J.; Sakagami, S.F. Australian stingless bees of the genus Trigona (Hymenoptera: Apidae). Invertebr. Taxon. 1997, 11, 861–896. [Google Scholar] [CrossRef]
- Rasmussen, C.; Cameron, S.A. A molecular phylogeny of the old world stingless bees (Hymenoptera: Apidae: Meliponini) and the non-monophyly of the large genus Trigona. Syst. Entomol. 2007, 32, 26–39. [Google Scholar] [CrossRef]
- Heard, T.A. The Australian Native Bee Book. In Keeping Stingless Bee Hives for Pets, Pollination and Sugarbag Honey; Sugarbag Bees: Brisbane, Australia, 2016. [Google Scholar]
- Roubik, D.W. Ecology and Natural History of Tropical Bees; Cambridge University Press: New York, NY, USA, 1989; p. 514. [Google Scholar]
- Michener, C.D. Observations on the nests and behavior of Trigona in Australia and New Guinea (Hymenoptera, Apidae). Am. Mus. Novit. 1961, 2026, 2–46. [Google Scholar]
- Brito, R.M.; Schaerf, T.M.; Myerscough, M.R.; Heard, T.A.; Oldroyd, B.P. Brood comb construction by the stingless bees Tetragonula hockingsi and Tetragonula carbonaria. Swarm Intell. 2012, 6, 151–176. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Niwor, CO, USA, 1993. [Google Scholar]
- De Groot, A.P. Protein and amino acid requirements of the honey bee (Apis mellifera L.). Physiol. Comp. Et Oecologia 1953, 3, 197–285. [Google Scholar]
- Moerman, R.; Vanderplanck, M.; Roger, N.; Declèves, S.; Wathelet, B.; Rasmont, P.; Fournier, D.; Michez, D. Growth rate of bumblebee larvae is related to pollen amino acids. J. Econ. Entomol. 2015, 109, 25–30. [Google Scholar] [CrossRef]
- Kriesell, L.; Hilpert, A.; Leonhardt, S.D. Different but the same. Bumblebee species collect pollen of different plant sources but similar amino acid profiles. Apidologie 2017, 48, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Rosumek, F.B.; Brückner, A.; Blüthgen, N.; Menzel, F.; Heethoff, M. Patterns and dynamics of neutral lipid fatty acids in ants—Implications for ecological studies. Front. Zool. 2017, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.L.; de Vere, N.; Keller, A.; Richardson, R.T.; Gous, A.; Burgess, K.S.; Brosi, B.J. Pollen DNA barcoding: Current applications and future prospects. Genome 2016, 59, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Sickel, W.; Ankenbrand, M.; Grimmer, G.; Holzschuh, A.; Härtel, S.; Lanzen, J.; Steffan-Dewenter, I.; Keller, A. Increased efficiency in identifying mixed pollen samples by meta-barcoding with a dual-indexing approach. BMC Ecol. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, A.; Danner, N.; Grimmer, G.; Ankenbrand, M.; von der Ohe, K.; von der Ohe, W.; Rost, S.; Härtel, S.; Steffan-Dewenter, I. Evaluating multiplexed next-generation sequencing as a method in palynology for mixed pollen samples. Plant Biol. 2015, 17, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the its2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Pcr Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: Cambridge, MA, USA, 1990; p. 428. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Global ITS2 Reference Database. Available online: https://zenodo.org/record/3344715#.XkaDo0oRXIU (accessed on 6 February 2020).
- Keller, A.; Hohlfeld, S.; Kolter, A.; Schultz, J.; Gemeinholzer, B.; Ankenbrand, M.J. Bcdatabaser: On-the-fly Reference Database Creation for (meta-)barcoding; EcoEvoRxiv: Würzburg, Germany, 2019. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.Org (accessed on 6 February 2020).
- Somerville, D.C. Lipid content of honey bee-collected pollen from south-east australia. Aust. J. Exp. Agr. 2005, 45, 1659–1661. [Google Scholar] [CrossRef]
- Somerville, D.C.; Nicol, H.I. Crude protein and amino acid composition of honeybee-collected pollen from south-east australia and a note on laboratory disparity. Aust. J. Exp. Agr. 2006, 46, 141–149. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Raubenheimer, D.; Kessner-Beierlein, D.; Grund-Mueller, N.; Noack, L.; Spaethe, J.; Leonhardt, S.D. Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecol. Lett. 2020, 23, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Manning, R. Fatty Acid Composition of Pollen and the Effect of Two Dominant Fatty Acids (linoleic and oleic) in Pollen and Flour Diets on Longevity and Nutritional Composition of Honey Bees (apis mellifera). Ph.D. Thesis, Murdoch University, Perth, Australia, 2006. [Google Scholar]
- Arien, Y.; Dag, A.; Shafir, S. Omega-6:3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. 2015, 112, 15761–15766. [Google Scholar] [CrossRef] [Green Version]
- Avni, D.; Hendriksma, H.P.; Dag, A.; Uni, Z.; Shafir, S. Nutritional aspects of honey bee-collected pollen and constraints on colony development in the eastern mediterranean. J. Insect Physiol. 2014, 69, 65–73. [Google Scholar] [CrossRef]
- Manning, R. Fatty acids in pollen: A review of their importance for honey bees. BeeWorld 2001, 82, 60–75. [Google Scholar] [CrossRef]
- Helm, B.R.; Slater, G.P.; Rajamohan, A.; Yocum, G.D.; Greenlee, K.J.; Bowsher, J.H. The geometric framework for nutrition reveals interactions between protein and carbohydrate during larval growth in honey bees. Biol. Open 2017, 6, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, S.H.; Jha, S. Wild bee nutritional ecology: Predicting pollinator population dynamics, movement and services from floral resources. Curr. Opin. Insect Sci. 2017, 21, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Pope, N.S.; Jha, S. Seasonal food scarcity prompts long-distance foraging by a wild social bee. Am. Naturalist 2018, 191, 45–57. [Google Scholar] [CrossRef] [PubMed]


| Habitat | GLM (Richness) | Spearman Correlation (Richness) | GLM (Habitat) | Tukey (Habitat, p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garden | Forest | Plantation | F | P | r | p | F | p | G-F | F-P | G-P | |
| Floral genus richness of LP | 16.00 ± 6.10 (13.29, 18.71) | 11.00 ± 6.76 (7.84, 14.16) | 9.43 ± 8.33 (4.62, 14.24) | 3.139 | 0.082 | 0.258 | 0.055 | 4.635 | 0.014 * | NS | NS | 0.021 ** |
| Sucrose (mg/mg LP) | 0.47 ± 0.20 (0.41, 0.54) | 0.57 ± 0.21 (0.50, 0.65) | 0.54 ± 0.22 (0.44, 0.64) | 5.565 | 0.021 * | −0.217 | 0.042 * | 2.016 | 0.140 | NA | NA | NA |
| Fat (µg/mg LP) | 7.22 ± 3.03 (5.91, 8.53) | 8.80 ± 6.94 (3.47, 14.13) | 9.50 ± 2.00 (7.97, 11.04) | 4.724 | 0.036 * | −0.354 | 0.023 * | 2.264 | 0.118 | NA | NA | NA |
| Protein (µg/mg LP) | 56.18 ± 24.42 (47.92, 64.44) | 63.65 ± 25.81 (54.10, 73.03) | 59.53 ± 31.66 (45.12, 73.94) | 0.164 | 0.686 | −0.046 | 0.670 | 0.815 | 0.446 | NA | NA | NA |
| Ratio S:P | 9.87 ± 6.35 (7.57, 11.55) | 8.38 ± 3.28 (8.30, 10.96) | 8.25 ± 3.52 (6.81, 16.13) | 2.675 | 0.106 | −0.177 | 0.098 | 0.386 | 0.681 | NA | NA | NA |
| Ratio S:F | 58.99 ± 33.32 (44.58, 73.39) | 46.46 ± 13.08 (36.41, 56.51) | 37.54 ± 16.51 (24.85, 50.23) | 1.778 | 0.191 | 0.150 | 0.361 | 1.991 | 0.151 | NA | NA | NA |
| Ratio P:F | 6.73 ± 2.92 (5.47, 7.99) | 6.25 ± 2.35 (4.44, 8.05) | 4.68 ± 1.39 (3.62, 5.75) | 3.590 | 0.066 | 0.304 | 0.053 | 2.072 | 0.140 | NA | NA | NA |
| Richness | Floral Richness of LP | Habitat | G-F | F-P | G-P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | |
| AA concentration (µg/mg LP) | 0.037 | 0.147 | 0.039 | 0.134 | 0.022 | 0.394 | NA | NA | NA | NA | NA | NA |
| AA mass proportion | 0.048 | 0.006 ** | 0.023 | 0.255 | 0.108 | <0.001 *** | NS | NS | 0.092 | 0.003 ** | 0.134 | <0.001 *** |
| FA concentration (µg/mg LP) | 0.087 | 0.026 * | 0.009 | 0.900 | 0.143 | 0.018 * | NS | NS | NS | NS | 0.178 | 0.005 ** |
| FA mass proportion | 0.078 | 0.063 | 0.016 | 0.725 | 0.297 | <0.001 *** | NS | NS | 0.342 | 0.008 ** | 0.326 | <0.001 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinkl, M.; Kaluza, B.F.; Wallace, H.; Heard, T.A.; Keller, A.; Leonhardt, S.D. Floral Species Richness Correlates with Changes in the Nutritional Quality of Larval Diets in a Stingless Bee. Insects 2020, 11, 125. https://doi.org/10.3390/insects11020125
Trinkl M, Kaluza BF, Wallace H, Heard TA, Keller A, Leonhardt SD. Floral Species Richness Correlates with Changes in the Nutritional Quality of Larval Diets in a Stingless Bee. Insects. 2020; 11(2):125. https://doi.org/10.3390/insects11020125
Chicago/Turabian StyleTrinkl, Moritz, Benjamin F. Kaluza, Helen Wallace, Tim A. Heard, Alexander Keller, and Sara D. Leonhardt. 2020. "Floral Species Richness Correlates with Changes in the Nutritional Quality of Larval Diets in a Stingless Bee" Insects 11, no. 2: 125. https://doi.org/10.3390/insects11020125
APA StyleTrinkl, M., Kaluza, B. F., Wallace, H., Heard, T. A., Keller, A., & Leonhardt, S. D. (2020). Floral Species Richness Correlates with Changes in the Nutritional Quality of Larval Diets in a Stingless Bee. Insects, 11(2), 125. https://doi.org/10.3390/insects11020125