Molecular Identification and Geometric Morphometric Analysis of Haematobosca aberrans (Diptera: Muscidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Specimen Collection and Identification
2.3. Molecular Analysis
2.3.1. DNA Extraction and COI Amplification
2.3.2. DNA Sequencing and Data Analysis
2.4. Geometric Morphometric Analysis
2.4.1. Wing Preparation
2.4.2. Landmark-Based Method
2.4.3. Morphometric Software
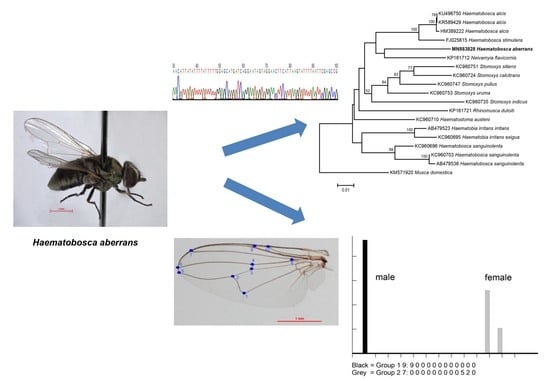
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zumpt, F. The Stomoxyine Biting Flies of the World; Gustav Fisher Verlag: Stuttgart, Germany, 1973; pp. 44–71. [Google Scholar]
- Burger, J.F.; Anderson, J.R. Taxonomy and life history of the Moose Fly, Haematobosca alcis, and its association with the moose, Alces alces shirasi in Yellowstone National Park. Ann. Ent. Soc. Am. 1974, 67, 204–214. [Google Scholar] [CrossRef]
- Pont, A.C.; Mihok, S. A new species of Haematobosca Bezzi from Kenya (Diptera, Muscidae). Stud. Dipterol. 2000, 7, 25–32. [Google Scholar]
- Pont, A.C.; Dsouli, N. A new species of Haematobosca Bezzi from Gabon (Diptera, Muscidae). Stud. Dipterol 2009, 15, 259–266. [Google Scholar]
- Changbunjong, T.; Weluwanarak, T.; Ratanakorn, P.; Maneeon, P.; Ganpanakngan, M.; Apiwathnasorn, C.; Sungvornyothin, S.; Sriwichai, P.; Sumruayphol, S.; Ruangsittichai, J. Distribution and abundance of Stomoxyini flies (Diptera: Muscidae) in Thailand. Southeast Asian J. Trop. Med. Public Health 2012, 43, 1400–1410. [Google Scholar] [PubMed]
- Pont, A.C.; Duvallet, G.; Changbunjong, T. A new species of Haematobosca Bezzi (Diptera: Muscidae) from Thailand. Zootaxa 2020, 4763, 538–544. [Google Scholar] [CrossRef]
- Tumrasvin, W.; Shinonaga, S. Studies on medically important flies in Thailand. V. On 32 species belonging to the subfamilies Muscinae and Stomoxyini including the taxonomic keys (Diptera: Muscidae). Bull. Tokyo Med. Dent. Univ. 1978, 25, 201–227. [Google Scholar]
- Hebert, P.D.N.; Gregory, T.R. The Promise of DNA Barcoding for Taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Meusnier, I.; Singer, G.A.; Landry, J.; Hickey, D.A.; Hebert, P.D.N.; Hajibabaei, M. A 683 universal DNA mini-barcode for biodiversity analysis. BMC Genom. 2008, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Pramual, P.; Adler, P.H. DNA barcoding of tropical black flies (Diptera: Simuliidae) of Thailand. Mol. Ecol. Resour. 2014, 14, 262–271. [Google Scholar] [CrossRef]
- Onder, Z.; Yildirim, A.; Duzlu, O.; Arslan, M.O.; Sari, B.; Tasci, G.T.; Ciloglu, A.; Aydin, N.P.; Inci, A.; Adler, P.H. Molecular characterization of black flies (Diptera: Simuliidae) in areas with pest outbreaks and simuliotoxicosis in Northeast Anatolia Region, Turkey. Acta Trop. 2019, 199, 105149. [Google Scholar] [CrossRef]
- Cywinska, A.; Hunter, F.F.; Hebert, P.D. Identifying Canadian mosquito species through DNA barcodes. Med. Vet. Entomol. 2006, 20, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Rajavel, A.R.; Natarajan, R.; Jambulingam, P. DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sumruayphol, S.; Apiwathnasorn, C.; Ruangsittichai, J.; Sriwichai, P.; Attrapadung, S.; Samung, Y.; Dujardin, J.P. DNA barcoding and wing morphometrics to distinguish three Aedes vectors in Thailand. Acta Trop. 2016, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vadivalagan, C.; Karthika, P.; Murugan, K.; Panneerselvam, C.; Del Serrone, P.; Benelli, G. Exploring genetic variation in haplotypes of the filariasis vector Culex quinquefasciatus (Diptera: Culicidae) through DNA barcoding. Acta Trop. 2017, 169, 43–50. [Google Scholar] [CrossRef]
- Contreras Gutiérrez, M.A.; Vivero, R.J.; Vélez, I.D.; Porter, C.H.; Uribe, S. DNA barcoding for the identification of sand fly species (Diptera, Psychodidae, Phlebotomine) in Colombia. PLoS ONE 2014, 9, e85496. [Google Scholar] [CrossRef]
- Nzelu, C.O.; Cáceres, A.G.; Arrunátegui-Jiménez, M.J.; Lañas-Rosas, M.F.; Yañez-Trujillano, H.H.; Luna-Caipo, D.V.; Holguín-Mauricci, C.E.; Katakura, K.; Hashiguchi, Y.; Kato, H. DNA barcoding for identification of sand fly species (Diptera: Psychodidae) from leishmaniasis-endemic areas of Peru. Acta Trop. 2015, 145, 45–51. [Google Scholar] [CrossRef]
- Romero-Ricardo, L.; Lastre-Meza, N.; Pérez-Doria, A.; Bejarano, E.E. DNA barcoding to identify species of phlebotomine sand fly (Diptera: Psychodidae) in the mixed leishmaniasis focus of the Colombian Caribbean. Acta Trop. 2016, 159, 125–131. [Google Scholar] [CrossRef]
- Lozano-Sardaneta, Y.N.; Paternina, L.E.; Sánchez-Montes, S.; Quintero, A.; Ibáñez-Bernal, S.; Sánchez-Cordero, V.; Bejarano, E.E.; Becker, I. DNA barcoding and fauna of phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) from Los Tuxtlas, Veracruz, Mexico. Acta Trop. 2020, 201, 105220. [Google Scholar] [CrossRef]
- Morita, S.I.; Bayless, K.M.; Yeates, D.K.; Wiegmann, B.M. Molecular phylogeny of the horse flies: A framework for renewing tabanid taxonomy. Syst. Entomol. 2016, 41, 56–72. [Google Scholar] [CrossRef]
- Nitiyamatawat, E.; Changbunjong, T.; Sumruayphol, S.; Sriwichai, P.; Thaenkham, U.; Ruangsittichai, J. DNA barcoding for identification of cleg flies, Haematopota spp. (Diptera: Tabanidae) in Thailand. Thai J. Vet. Med. Suppl. 2017, 47, 303–305. [Google Scholar]
- Changbunjong, T.; Bhusri, B.; Sedwisai, P.; Weluwanarak, T.; Nitiyamatawat, E.; Chareonviriyaphap, T.; Ruangsittichai, J. Species identification of horse flies (Diptera: Tabanidae) in Thailand using DNA barcoding. Vet. Parasitol. 2018, 259, 35–43. [Google Scholar] [CrossRef]
- Changbunjong, T.; Weluwanarak, T.; Sedwisai, P.; Ruangsittichai, J.; Duvallet, G.; Chareonviriyaphap, T. New records and DNA barcoding of deer flies, Chrysops (Diptera: Tabanidae) in Thailand. Acta Trop. 2020, 210, 105532. [Google Scholar] [CrossRef] [PubMed]
- Changbunjong, T.; Weluwanarak, T.; Samung, Y.; Ruangsittichai, J. Molecular identification and genetic variation of stomoxyine flies (Diptera: Muscidae) in Thailand based on cox1 barcode. J. Asia Pac. Entomol. 2016, 19, 1117–1123. [Google Scholar] [CrossRef]
- Dujardin, J.P. Morphometrics applied to medical entomology. Infect. Genet. Evol. 2008, 8, 875–890. [Google Scholar] [CrossRef]
- Changbunjong, T.; Sumruayphol, S.; Weluwanarak, T.; Ruangsittichai, J.; Dujardin, J.P. Landmark and outline-based geometric morphometrics analysis of three Stomoxys flies (Diptera: Muscidae). Folia Parasitol. (Praha) 2016, 63, 37. [Google Scholar]
- Lorenz, C.; Almeida, F.; Almeida-Lopes, F.; Louise, C.; Pereira, S.N.; Petersen, V.; Vidal, P.O.; Virginio, F.; Suesdek, L. Geometric morphometrics in mosquitoes: What has been measured? Infect. Genet. Evol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Sontigun, N.; Sukontason, K.L.; Zajac, B.K.; Zehner, R.; Sukontason, K.; Wannasan, A.; Amendt, J. Wing morphometrics as a tool in species identification of forensically important blow flies of Thailand. Parasit. Vectors 2017, 10, 229. [Google Scholar] [PubMed] [Green Version]
- Chaiphongpachara, T. Comparison of Landmark- and Outline-Based Geometric Morphometrics for Discriminating Mosquito Vectors in Ratchaburi Province, Thailand. Biomed. Res. Int. 2018, 2018, 6170502. [Google Scholar] [CrossRef] [PubMed]
- Chaiphongpachara, T.; Sriwichai, P.; Samung, Y.; Ruangsittichai, J.; Morales Vargas, R.E.; Cui, L.; Sattabongkot, J.; Dujardin, J.P.; Sumruayphol, S. Geometric morphometrics approach towards discrimination of three member species of Maculatus group in Thailand. Acta Trop. 2019, 192, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.P.; Kaba, D.; Solano, P.; Dupraz, M.; McCoy, K.D.; Jaramillo-O, N. Outline-based morphometrics, an overlooked method in arthropod studies? Infect. Genet. Evol. 2014, 28, 704–714. [Google Scholar]
- Chaiphongpachara, T.; Laojun, S. Using the modern morphometric approach to determine sexual dimorphism of three medically important flies (Order: Diptera) in Thailand. Biodiversitas 2019, 20, 1482–1486. [Google Scholar] [CrossRef]
- Ruangsittichai, J.; Apiwathnasorn, C.; Dujardin, J.P. Interspecific and sexual shape variation in the filariasis vectors Mansonia dives and Ma. bonneae. Infect. Genet. Evol. 2011, 11, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Virginio, F.; Oliveira Vidal, P.; Suesdek, L. Wing sexual dimorphism of pathogen-vector culicids. Parasit. Vectors 2015, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, W.R.; de Camargo, N.F.; Corrêa, D.; de Camargo, A.J.; Diniz, I.R. Sexual Dimorphism and Allometric Effects Associated with the Wing Shape of Seven Moth Species of Sphingidae (Lepidoptera: Bombycoidea). J. Insect Sci. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Benítez, H.A.; Vargas, H.A. Sexual dimorphism and population differentiation in the Chilean Neotropical moth Macaria mirthae (Lepidoptera, Geometridae): A wing geometric morphometric example. Rev. Bras. Entomol. 2017, 61, 365–369. [Google Scholar] [CrossRef]
- Gidaszewski, N.A.; Baylac, M.; Klingenberg, C.P. Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evol. Biol. 2009, 9, 110. [Google Scholar] [CrossRef]
- Mihok, S. The development of a multipurpose trap (the Nzi) for tsetse and other biting flies. Bull. Entomol. Res. 2002, 92, 385–403. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, M.; Ishiguro, N. Genetic and morphological differences of Haematobia irritans and H. exigua and molecular phylogeny of Japanese Stomoxyini flies (Diptera, Muscidae). Med. Entomol. Zool. 2010, 61, 335–344. [Google Scholar] [CrossRef]
- Renaud, A.K.; Savage, J.; Adamowicz, S.J. DNA barcoding of Northern Nearctic Muscidae (Diptera) reveals high correspondence between morphological and molecular species limits. BMC Ecol. 2012, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; deWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef] [Green Version]
- Sikes, D.S.; Bowser, M.; Morton, J.M.; Bickford, C.; Meierotto, S.; Hildebrandt, K. Building a DNA barcode library of Alaska’s non-marine arthropods. Genome 2017, 60, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.N.; Pape, T.; Pont, A.; Wiegmann, B.M.; Meier, R. The Muscoidea (Diptera: Calyptratae) are paraphyletic: Evidence from four mitochondrial and four nuclear genes. Mol. Phylogenet. Evol. 2008, 49, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Haseyama, K.L.; Wiegmann, B.M.; Almeida, E.A.; de Carvalho, C.J. Say goodbye to tribes in the new house fly classification: A new molecular phylogenetic analysis and an updated biogeographical narrative for the Muscidae (Diptera). Mol. Phylogenet. Evol. 2015, 89, 1–12. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology, 1st ed.; Cambridge University Press: New York, NY, USA, 1991; 435p. [Google Scholar]
- Rohlf, F.J. Rotational fit (Procrustes) methods. In Proceedings of the Michigan Morphometrics Workshop; Rohlf, F.J., Bookstein, F.L., Eds.; The University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1990; pp. 227–236. [Google Scholar]
- Changbunjong, T.; Weluwanarak, T.; Sedwisai, P.; Chamsai, T. Stomoxyini fly fauna of the Khao Yai National Park, Thailand. Asian Pac. J. Trop. Dis. 2013, 3, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef]







| Species | Origin | Accession Number | Reference |
|---|---|---|---|
| Haematobia irritans irritans (Linnaeus, 1758) | Japan | AB479523 | [41] |
| Haematobia irritans exigua de Meijere, 1906 | Thailand | KC960695 | [24] |
| Haematobosca alcis (Snow, 1891) | Canada | HM389222, KR589429 | [42,43] |
| USA | KU496750 | [44] | |
| Haematobosca sanguinolenta (Austen, 1909) | Thailand | KC960696, KC960703 | [24] |
| Vietnam | AB479536 | [41] | |
| Haematobosca stimulans (Meigen, 1824) | NA | FJ025615 | [45] |
| Haematostoma austeni Malloch, 1932 | Thailand | KC960710 | [24] |
| Neivamyia flavicornis (Malloch, 1928) | Brazil | KP161712 | [46] |
| Rhinomusca dutoiti Zumpt, 1950 | South Africa | KP161721 | [46] |
| Stomoxys calcitrans (Linnaeus, 1758) | Thailand | KC960724 | [24] |
| Stomoxys indicus Picard, 1908 | Thailand | KC960735 | [24] |
| Stomoxys pullus Austen, 1909 | Thailand | KC960747 | [24] |
| Stomoxys sitiens Rondani, 1873 | Thailand | KC960751 | [24] |
| Stomoxys uruma Shinonaga & Kano, 1966 | Thailand | KC960753 | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changbunjong, T.; Ruangsittichai, J.; Duvallet, G.; Pont, A.C. Molecular Identification and Geometric Morphometric Analysis of Haematobosca aberrans (Diptera: Muscidae). Insects 2020, 11, 451. https://doi.org/10.3390/insects11070451
Changbunjong T, Ruangsittichai J, Duvallet G, Pont AC. Molecular Identification and Geometric Morphometric Analysis of Haematobosca aberrans (Diptera: Muscidae). Insects. 2020; 11(7):451. https://doi.org/10.3390/insects11070451
Chicago/Turabian StyleChangbunjong, Tanasak, Jiraporn Ruangsittichai, Gerard Duvallet, and Adrian C. Pont. 2020. "Molecular Identification and Geometric Morphometric Analysis of Haematobosca aberrans (Diptera: Muscidae)" Insects 11, no. 7: 451. https://doi.org/10.3390/insects11070451
APA StyleChangbunjong, T., Ruangsittichai, J., Duvallet, G., & Pont, A. C. (2020). Molecular Identification and Geometric Morphometric Analysis of Haematobosca aberrans (Diptera: Muscidae). Insects, 11(7), 451. https://doi.org/10.3390/insects11070451