Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Period and Sites
2.2. Egg Location
2.3. Spatial Pattern and Sequential Plans
2.4. Data Analysis
2.4.1. Egg Location
2.4.2. Spatial Pattern and Sequential Plan
3. Results
3.1. Egg Location
3.1.1. Tomato Fruitworm Eggs
3.1.2. Plusiinae Eggs
3.1.3. Egg Location—Comparison between Helicoverpa armigera and Plusiinae
3.1.4. Egg Location—Comparison between Parasitized vs. Non-Parasitized Eggs
3.2. Spatial Pattern in the Field
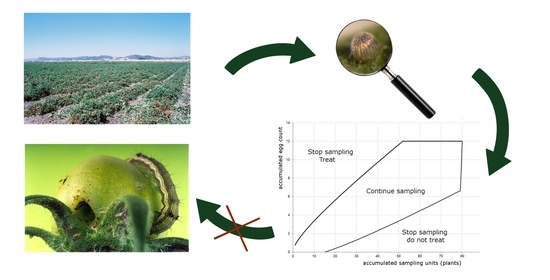
3.3. Sequential Plans
3.3.1. Sequential Plan for Tomato Fruitworm Egg Density Classification and Its Validation
3.3.2. Sequential Plan for Tomato Fruitworm Egg Density Estimation and Its Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colvine, S. WPTC Crop Update as of 28 January 2020. Available online: http://www.tomatonews.com/en/wptc-crop-update-as-of-28-january-2020_2_925.html (accessed on 8 August 2020).
- Agro.Ges. Fileira do tomate de indústria. Millenium Agro News 2019, 2, 3–15. Available online: https://ind.millenniumbcp.pt/pt/negocios/financiamento/Documents/Millennium_Agro_News_dez19.pdf (accessed on 21 July 2020).
- INE. Estatísticas Agrícolas 2018; Instituto Nacional de Estatística, I.P.: Lisbon, Portugal, 2019; p. 168. [Google Scholar]
- Pinto, F.A.; Mattos, M.V.V.; Silva, F.W.S.; Rocha, S.L.; Elliot, S.L. The spread of Helicoverpa armigera. Insects 2017, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Burgio, G.; Ravaglia, F.; Maini, S.; Bazzocchi, G.G.; Masetti, A.; Lanzoni, A. Mating disruption of Helicoverpa armigera (Lepidoptera: Noctuidae) on processing tomato: First applications in Northern Italy. Insects 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammers, J.W.; Macleod, A. Report of a Pest Risk Analysis: Helicoverpa armigera (Hübner, 1808); Plant Protection Service & Central Science Laboratory: Wageningen, The Netherlands; York, UK, 2007; p. 18. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/downloadExternalPra.cfm?id=3879 (accessed on 8 August 2020).
- Meierrose, C.; Araújo, J.; Perkins, D.; Mercadier, G.; Poitout, S.; Bues, R.; Vargas-Piqueras, P.; Cabello, T. Distribution and economic importance of Heliothis spp. (Lep.: Noctuidae) and their natural enemies and host plants in Western Europe. In Proceedings of the Workshop Biological Control of Heliothis: Increasing Effectiveness of Natural Enemies; New Delhi, India, 11–15 November 1985; King, E.G., Jackson, R.D., Eds.; India Far Eastern Regional Research Office, U.S. Department of Agriculture: New Delhi, India, 1989; pp. 311–327. [Google Scholar]
- Singh, N.; Dotasara, S.K.; Jat, S.M.; Naqvi, A.R. Assessment of crop losses due to tomato fruit borer, Helicoverpa armigera in tomato. J. Entomol. Zool. Stud. 2017, 5, 595–597. [Google Scholar]
- Reddy, G.V.P.; Manjunatha, M. Laboratory and field studies on the integrated pest management of Helicoverpa armigera (Hubner) in cotton, based on pheromone trap catch threshold level. J. Appl. Entomol. 2000, 124, 213–221. [Google Scholar] [CrossRef]
- Nilakhe, S.S.; Chalfant, R.B.; Phatak, S.C.; Mullinix, B. Tomato fruitworm: Development of sequential sampling and comparison with conventional sampling in tomatoes. J. Econ. Entomol. 1982, 75, 416–421. [Google Scholar] [CrossRef]
- Bolkan, H.A.; Reinert, W.R. Developing and implementing IPM strategies to assist farmers: An industry approach. Plant Dis. 1994, 78, 545–550. [Google Scholar] [CrossRef]
- Zalom, F.G.; Weakley, C.V.; Hoffmann, M.P.; Wilson, L.T.; Grieshop, J.I.; Miyao, G. Monitoring tomato fruitworm eggs in processing tomatoes. Calif. Agric. 1990, 44, 12–15. [Google Scholar] [CrossRef]
- Dawson, J.; Hamilton, A.J.; Mansfield, C. Dispersion statistics and a sampling plan for Helicoverpa (Lepidoptera: Noctuidae) on fresh-market tomatoes (Lycopersicon esculentum). Aust. J. Entomol. 2006, 45, 91–95. [Google Scholar] [CrossRef]
- Terry, I.; Bradley, J.R.J.; van Duyn, J.W. Heliothis zea (Lepidoptera: Noctuidae) eggs in soybeans: Within-field distribution and precision level sequential count plans. Environ. Entomol. 1989, 18, 908–916. [Google Scholar] [CrossRef]
- Gozé, E.; Nibouche, S.; Deguine, J.P. Spatial and probability distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in cotton: Systematic sampling, exact confidence intervals and sequential test. Environ. Entomol. 2003, 32, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, P.K.; Hutchison, W.D. Sequential sampling plans for estimating European corn borer (Lepidoptera: Crambidae) and corn earworm (Lepidoptera: Noctuidae) larval density in sweet corn ears. Crop Prot. 2003, 22, 903–909. [Google Scholar] [CrossRef]
- Beyo, J.; Nibouche, S.; Gozé, E.; Deguine, J.P. Application of probability distribution to the sampling of cotton bollworms (Lepidoptera: Noctuidae) in Northern Cameroon. Crop Prot. 2004, 23, 1111–1117. [Google Scholar] [CrossRef]
- Walker, G.P.; Herman, T.J.B.; Kale, A.J.; Wallace, A.R. An adjustable action threshold using larval parasitism of Helicoverpa armigera (Lepidoptera: Noctuidae) in IPM for processing tomatoes. Biol. Control 2010, 52, 30–36. [Google Scholar] [CrossRef]
- Wilson, L.T.; Zalom, F.G.; Smith, R.; Hoffmann, M.P. Monitoring for fruit damage in processing tomatoes: Use of a dynamic sequential sampling plan. Environ. Entomol. 1983, 12, 835–839. [Google Scholar] [CrossRef]
- Bouchard, D.; Ouedrogo, A.; Boivin, G. Vertical distribution, spatial dispersion and sequential sampling plan for fruit damage by Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on tomato crop in Burkina Faso. Trop. Pest Manag. 1992, 38, 250–253. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Lacasa-Plasencia, A. Impact of Helicoverpa armigera larval density and crop phenology on yield and quality losses in processing tomato: Developing fruit count-based damage thresholds for IPM decision-making. Crop Prot. 2003, 22, 521–532. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Lacasa-Plasencia, A. Testing IPM protocols for Helicoverpa armigera in processing tomato: Egg-count vs. fruit-count-based damage thresholds using Bt or chemical insecticides. Crop Prot. 2003, 22, 1045–1052. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Nault, B.A.; Hitchner, E.M.; Speese, J. Evaluation of action threshold-based insecticide spray programs for tomato fruitworm management in fresh-market tomatoes in Virginia. Crop Prot. 2006, 25, 604–612. [Google Scholar] [CrossRef]
- Cameron, P.J.; Walker, G.P.; Hodson, A.J.; Kale, A.J.; Herman, T.J.B. Trends in IPM and insecticide use in processing tomatoes in New Zealand. Crop Prot. 2009, 28, 421–427. [Google Scholar] [CrossRef]
- Gonçalves, C.I.; Amaro, F.; Figueiredo, E.; Godinho, M.; Mexia, A. Estudo da aceitação e adequabilidade dos ovos de algumas espécies de noctuídeos como hospedeiros de Trichogramma sp. (Hymenoptera: Trichogrammatidae). In Proceedings of the 6o Encontro Nacional Protecção Integrada, Castelo Branco, Portugal, 14–16 May 2003; ESACB: Castelo Branco, Portugal, 2005; pp. 515–521. [Google Scholar]
- Figueiredo, E.; Amaro, F.; Godinho, M.; Stilwell, S.; Albano, S.; Salvado, E.; Mexia, A. Protecção Integrada em tomate de indústria-estimativa do risco de lagarta do tomate Helicoverpa armigera (Hbn.). In Proceedings of the 6o Encontro Nacional Protecção Integrada, Castelo Branco, 14–16 May 2003; ESACB: Castelo Branco, Portugal, 2005; pp. 557–564. [Google Scholar]
- Figueiredo, E.; Amaro, F.; Gonçalves, C.; Godinho, M.C.; Salvado, E.; Albano, S. Lagarta do tomate. In Protecção Integrada em Tomate de Indústria; Amaro, F., Mexia, A., Eds.; EAN/INIAP: Oeiras, Portugal, 2006; pp. 42–50. [Google Scholar]
- Cameron, P.J.; Walker, G.P.; Herman, T.J.B.; Wallace, A.R. Development of economic thresholds and monitoring systems for Helicoverpa armigera (Lepidoptera: Noctuidae) in tomatoes. J. Econ. Entomol. 2001, 94, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, G.E.; Fitt, G.P. Reassessment of sampling relationships for Helicoverpa spp. (Lepidoptera: Noctuidae) in Australian cotton. Aust. J. Entomol. 1995, 40, 151–157. [Google Scholar] [CrossRef]
- Mabbett, T.H.; Nachapong, M. Within-plant distribution of Heliothis armigera eggs on cotton in Thailand. Trop. Pest Manag. 1984, 30, 367–371. [Google Scholar] [CrossRef]
- Farrar, R.R.; Bradley, J.R. Within-plant distribution of Heliothis spp. (Lepidoptera: Noctuidae) eggs and larvae on cotton in North Carolina. Environ. Entomol. 1985, 14, 205–209. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Wilson, L.T.; Blood, P.R.B. Oviposition by Heliothis armigera and H. punctigera (Lepidoptera: Noctuidae) on okra leaf and smooth-leaf cotton. Environ. Entomol. 1990, 19, 710–716. [Google Scholar] [CrossRef]
- Braswell, L.R.; Reisig, D.D.; Sorenson, C.E.; Collins, G.D. Field and forage crops Helicoverpa zea (Lepidoptera: Noctuidae) oviposition and larval vertical distribution in Bt cotton under different levels of nitrogen and irrigation. J. Econ. Entomol. 2020, 112, 1237–1250. [Google Scholar] [CrossRef]
- Duffield, S.J.; Chapple, D.G. Within-plant distribution of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) eggs on irrigated soybean. Aust. J. Entomol. 2001, 40, 151–157. [Google Scholar] [CrossRef]
- Van den Berg, H.; Cock, M.J.W. Spatial association between Helicoverpa armigera and its predators in smallholder crops in Kenya. J. Appl. Ecol. 1995, 32, 242–252. [Google Scholar] [CrossRef]
- Izquierdo, J.; Arilla, E.; Ramirez, M.; Abad, J. Plusiinae (Lépidoptera: Noctuidae) en tomate: Especies, evolución en la campaña y distribución en la planta. Bol. Sanidade Veg. Plagas 1996, 22, 803–810. [Google Scholar]
- Izquierdo, J.; Figueras, M. Niveles y distribución de la puesta de Helicoverpa armigera (Hbn.) en cultivo de tomate exterior para consumo en fresco. Investig. Agrárias Prod. Protección Veg. 1993, 8, 431–443. [Google Scholar]
- CABI. Helicoverpa armigera. In Invasive Species Compendium; CABI International: Wallingford, UK, 2019; Available online: https://www.cabi.org/isc/datasheet/26757#todetectionAndInspection (accessed on 18 December 2019).
- Alvarado-Rodriguez, B.; Leigh, T.F.; Lange, W.H. Oviposition site preference by the tomato fruitworm (Lepidoptera: Noctuidae) on tomato, with notes on plant phenology. J. Econ. Entomol. 1982, 75, 895–898. [Google Scholar] [CrossRef]
- Snodderly, L.J.; Lambdin, P. Oviposition and feeding sites of Heliothis zea on tomato. Environ. Entomol. 1982, 11, 513–515. [Google Scholar] [CrossRef]
- Gonçalves, C.I.; Huigens, M.E.; Verbaarschot, P.; Duarte, S.; Mexia, A.; Tavares, J. Natural occurrence of Wolbachia-infected and uninfected Trichogramma species in tomato fields in Portugal. Biol. Control 2006, 37, 375–381. [Google Scholar] [CrossRef]
- Hollingsworth, C.S.; Gatsonis, C. Sequential sampling plans for green peach aphid (Homoptera: Aphididae) on potato. J. Econ. Entomol. 1990, 83, 1365–1369. [Google Scholar] [CrossRef]
- Binns, M. Sequential sampling for classifying pest status. In Handbook of Sampling Methods for Arthropods in Agriculture; Pedigo, L.P., Buntin, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 137–174. [Google Scholar]
- Nyrop, J.P.; Binns, M.R.; van der Werf, W. Sampling for IPM decision-making: Where should we invest time and resources. Phytopathology 1999, 89, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Binns, M.R.; Nyrop, J.P.; van der Werf, W. Sampling and Monitoring in Crop Protection. The Theoretical Basis for Developing Practical Decision Guides; CABI Publishing: Wallingford, UK, 2000; p. 304. [Google Scholar]
- Debouzie, D.; Thioulouse, J. Statistics to find spatial and temporal structures in populations. In Pest Control: Operations and Systems Analysis in Fruit Fly Management; Mangel, M., Carey, J.R., Plant, R.E., Eds.; Springer: New York, NY, USA, 1986; pp. 263–282. [Google Scholar]
- Ho, C.-C. Dispersion statistics and sample size estimates for Tetranychus kanzawai (Acari: Tetranychidae) on mulberry. Environ. Entomol. 1993, 22, 21–25. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science: Oxford, UK, 2000; p. 575. [Google Scholar]
- Pedigo, L.P. Entomology and Pest Management, 2nd ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1996; p. 667. [Google Scholar]
- IBM Corp IBM SPSS Statistics for Windows, Version 24.0. 2016. Available online: https://www.ibm.com/products/spss- (accessed on 28 December 2019).
- Sawada, M. Geometric Mean Regression Add-In for Excel ’97. Available online: http://www.lpc.uottawa.ca/data/scripts/index.html (accessed on 13 August 2019).
- Taylor, L.R. Assessing and interpreting the spatial distributions of insect populations. Annu. Rev. Entomol. 1984, 29, 321–357. [Google Scholar] [CrossRef]
- Arbab, A.; Mcneill, M.R. Spatial distribution and sequential sampling plans for adult Sitona humeralis Stephens (Coleoptera: Curculionidae) in alfalfa. J. Asia Pac. Entomol. 2014, 17, 515–519. [Google Scholar] [CrossRef]
- Shahbi, M.; Rajabpour, A. A fixed-precision sequential sampling plan for the potato tuberworm moth, Phthorimaea operculella Zeller (Lepidoptera: Gelechidae), on potato cultivars. Neotrop. Entomol. 2017, 46, 388–395. [Google Scholar] [CrossRef]
- Maroco, J.P. Análise Estatística com o SPSS Statistics, 5th ed.; ReportNumber: Pêro Pinheiro, Portugal, 2011; p. 990. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Singapore, 1984; p. 680. [Google Scholar]
- Faulster, D.S.; Warton, D.I.; Wright, I.J. (S)MATR: Standardised major axis tests and routines, Version 1.0. 2003. Available online: http://www.bio.mq.edu.au/ecology/SMATR(accessed on 28 December 2020).
- Binns, M.R.; Nyrop, J.P. Sampling insect populations for the purpose of IPM decision making. Annu. Rev. Entomol. 1992, 37, 427–453. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harpin & Row Publishers: New York, NY, USA, 1989; p. 654. [Google Scholar]
- Hutchison, W. Sequential sampling to determine population density. In Handbook of Sampling Methods for Arthropods in Agriculture; Pedigo, L.P., Buntin, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 207–243. [Google Scholar]
- Naranjo, S.E.; Hutchison, W.D. RVSP2—Resampling Software for Analysis and Validation of Enumerative and Binomial Sampling Plans. 2003. Available online: http://www.ars.usda.gov/services/software/download.htm?softwareid=129 (accessed on 8 January 2007).
- Damos, P. Density-invariant dispersion indices and fixed precision sequential sampling plans for the peach twig borer Anarsia lineatella (Lepidoptera: Gelechiidae). Eur. J. Entomol. 2018, 115, 642–649. [Google Scholar] [CrossRef]
- Pezzini, D.T.; DiFonzo, C.D.; Finke, D.L.; Hunt, T.E.; Knodel, J.J.; Krupke, C.H.; McCornack, B.; Michel, A.P.; Moon, R.D.; Philips, C.R.; et al. Spatial patterns and sequential sampling plans for estimating densities of stink bugs (Hemiptera: Pentatomidae) in soybean in the north central region of the United States. J. Econ. Entomol. 2019, 112, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Zalom, F.G.; Wilson, L.T.; Smith, R. Oviposition patterns by several lepidopterous pests on processing tomatoes in California. Environ. Entomol. 1983, 12, 1133–1137. [Google Scholar] [CrossRef]
- Wilcox, J.; Howland, A.F.; Campbell, R.E. Investigations of the tomato fruitworm, its seasonal history and methods of control. U.S. Dep. Agric. Tech. Bull. 1956, 1147, 47. [Google Scholar]
- Zitsanza, E.S.; Giga, D.P.; Knight, J.D. Oviposition site preferences by Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of season and density on distribution of eggs on plants. South African J. Plant Soil 2006, 23, 138–141. [Google Scholar] [CrossRef]
- Saour, G.; Causse, R. Comportement de ponte d’ Heliothis armigera Hübner (Lep., Noctuidae) sur tomate. J. Appl. Entomol. 1993, 115, 203–209. [Google Scholar] [CrossRef]
- Araújo, A.C.M. Luta Biológica contra Heliothis armigera no Ecossistema Agrícola “Tomate para Indústria”. Interacções Cultura-Fitófagos-Antagonistas. Ph.D. Thesis, University Évora, Évora, Portugal, 1990. [Google Scholar]
- King, A.B. Heliothis/Helicoverpa (Lepidoptera: Noctuidae). In Insect Pests of Cotton; Mattews, G., Tunstall, J., Eds.; CAB International: Wallingford, UK, 1994; pp. 39–106. [Google Scholar]
- Taylor, L.R.; Woiwod, I.P.; Perry, J. The negative binomial as a dynamic ecological model for aggregation, and the density dependence of k. J. Anim. Ecol. 1979, 48, 289–304. [Google Scholar] [CrossRef]
- Perry, J.N.; Woiwod, I.P. Fitting Taylor’s power law. Oikos 1992, 65, 538–542. [Google Scholar] [CrossRef]
- Hoffmann, M.P.; Wilson, L.T.; Zalom, F.G.; Hilton, R. Dynamic sequential sampling plan for Helicoverpa zea (Lepidoptera: Noctuidae) eggs in processing tomatoes: Parasitism and temporal patterns. Environ. Entomol. 1991, 20, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.T.; Room, P.M. Clumping patterns of fruit and arthropods in cotton, with implications for binomial sampling. Environ. Entomol. 1983, 12, 50–54. [Google Scholar] [CrossRef]
- Kring, T.J.; Yearian, W.C.; Steward, V.B. Within-field and within-panicle distribution of Heliothis zea (Lepidoptera: Noctuidae) and Celama sorgiella (Lepidoptera: Noctuidae) eggs in grain sorghum. Environ. Entomol. 1989, 18, 150–156. [Google Scholar] [CrossRef]
- Nyrop, J.P.; Reissig, W.H.; Agnello, A.M.; Kovach, J. Development and evaluation of a control decision rule for first generation spotted tentiform leafminer (Lepidoptera: Gracillariidae) in New York apple orchards. Environ. Entomol. 1990, 19, 1624–1638. [Google Scholar] [CrossRef] [Green Version]
- Cullen, E.M.; Zalom, F.G.; Flint, M.L.; Zilbert, E.E. Quantifying trade-offs between pest sampling time and precision in commercial IPM sampling programs. Agric. Syst. 2000, 66, 99–113. [Google Scholar] [CrossRef]
- Hoffmann, M.P.; Wilson, L.T.; Zalom, F.G.; Hilton, R. Parasitism of Heliothis zea (Lepidoptera: Noctuidae) eggs: Effect on pest management decision rules for processing tomatoes in the Sacramento Valley of California. Environ. Entomol. 1990, 19, 753–763. [Google Scholar] [CrossRef]
- Ortiz-García, R.; Barreiro-García, J. Control integrado de plagas de tomate en las Vegas del Guadiana. Bol. Sanidade Veg. Plagas 1994, 20, 243–246. [Google Scholar]



| Plant Part | Position | Mean Proportion of Eggs of (±SE) (%) 2 | Parasitism—Mean Proportion of Eggs (±SE) (%) 3 | ||
|---|---|---|---|---|---|
| H. armigera | Plusiinae | Non-Parasitized | Parasitized | ||
| Leaf | a | 39.4 ± 3.3 a | 16.9 ± 3.1 b | 38.3± 3.2 A | 28.7± 3.0 A |
| b | 40.3 ± 3.1 a | 45.0 ± 4.2 a | 39.7± 3.4 A | 40.1 ± 2.9 A | |
| c | 19.5 ± 2.4 a | 38.2 ± 4.3 b | 26.4 ± 3.0 A | 31.2 ± 3.4 A | |
| Leaflet | 1 | 33.6 ± 3.2 a | 42.7 ± 4.7 a | 33.2 ± 3.0 A | 42.5 ± 3.3 B |
| 2 | 36.1 ± 3.2 a | 30.7 ± 4.2 a | 33.1 ± 3.0 A | 38.5 ± 4.1 A | |
| 3 | 24.4 ± 1.6 a | 24.8 ± 4.0 a | 26.5 ± 2.8 A | 16.5 ± 2.4 B | |
| 4 | 6.7 ± 1.6 a | 1.8 ± 0.9 b | 6.9 ± 1.8 A 5 | 2.4 ± 0.8 A 5 | |
| 5 | 0.2 ± 0.1 a | 0.0 ± 0.0 a | 0.3 ± 0.3 A | 0.3 ± 0.2 A | |
| Leaf side | lower | 79.0 ± 2.4 a | 86.0 ± 3.4 b | 81.8 ± 2.9 A 4 | 75.1 ± 3.7 A 4 |
| upper | 21.0 ± 2.4 a | 14.0 ± 3.4 b | 24.6 ± 2.9 A 4 | 15.7 ± 2.6 A 4 | |
| Plant Part | Location | Egg Mean Number per Observation Date (±SE) 2 | |
|---|---|---|---|
| Helicoverpa armigera | Plusiinae | ||
| Leaf | a | 4.16 ± 0.65 a | 0.80 ± 0.18 A |
| b | 4.37 ± 0.58 a | 1.90 ± 0.28 B | |
| c | 2.46 ± 0.41 b | 1.38 ± 0.24 B | |
| Leaflet | 1 | 4.16 ± 0.61 a | 1.68 ± 0.26 A |
| 2 | 3.69 ± 0.57 a | 1.30 ± 0.23 A | |
| 3 | 2.63 ± 0.40 a | 0.87 ± 0.13 A | |
| 4 | 0.70 ± 0.13 b | 0.09 ± 0.03 B | |
| 5 | 0.06 ± 0.04 b | 0.00 ± 0.00 B | |
| Leaf side | lower | 7.77 ± 0.92 a | 3.77 ± 0.57 A |
| upper | 3.48 ± 0.67 b | 0.45 ± 0.10 B | |
| Phenological Stage | Mean ± SE | Friedman Test | ||||
|---|---|---|---|---|---|---|
| a | b | c | N | χ2 | p | |
| Flowering | 4.96 ± 1.44 ab | 4.78 ± 1.02 a | 2.28 ± 0.64 b | 28 | 12.9 | 0.002 |
| Green fruit | 3.70 ± 0.98 a | 4.44 ± 1.26 a | 2.59 ± 0.87 a | 27 | 3.34 | 0.188 |
| Flowering + green fruit | 4.34 ± 0.87 a | 4.61 ± 0.80 a | 2.43 ± 0.57 b | 55 | 14.9 | 0.001 |
| Maturing-senescence | 3.97 ± 0.95 a | 4.06 ± 0.78 a | 2.58 ± 0.68 a | 31 | 7.02 | 0.03 |
| TPL | N | Range Means | b(1) | SE b | a | Confidence Interval for b | r2 (2) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% | 99% | |||||||||
| Model | 105 | 0.03–1.26 | 1.137 *** | 0.039 | 1.810 | 1.063 | 1.217 | 1.04 | 1.244 | 0.879 |
| Leziria Grande | 72 | 0.02–1.26 | 1.107 * | 0.047 | 1.696 | 1.017 | 1.206 | 0.989 | 1.24 | 0.871 |
| Valada do Ribatejo | 43 | 0.03–0.48 | 1.339 *** | 0.077 | 3.184 | 1.180 | 1.498 | 1.126 | 1.552 | 0.858 |
| Canha + Coruche | 24 | 0.03–0.88 | 1.279 * | 0.112 | 2.630 | 1.058 | 1.546 | 0.990 | 1.653 | 0.814 |
| 2002 | 11 | 0.02–0.47 | 1.196 * | 0.077 | 2.118 | 1.019 | 1.403 | 0.952 | 1.503 | 0.955 |
| 2003 | 49 | 0.05–1.26 | 1.161 * | 0.070 | 1.887 | 1.026 | 1.314 | 0.985 | 1.369 | 0.821 |
| 2004 | 79 | 0.03–1.26 | 1.164 *** | 0.048 | 1.977 | 1.072 | 1.262 | 1.044 | 1.296 | 0.871 |
| Validation fields | 34 | 0.02–1.26 | 1.181 ** | 0.063 | 2.155 | 1.056 | 1.321 | 1.016 | 1.373 | 0.902 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, E.; Gonçalves, C.; Duarte, S.; Godinho, M.C.; Mexia, A.; Torres, L. Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling. Insects 2021, 12, 13. https://doi.org/10.3390/insects12010013
Figueiredo E, Gonçalves C, Duarte S, Godinho MC, Mexia A, Torres L. Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling. Insects. 2021; 12(1):13. https://doi.org/10.3390/insects12010013
Chicago/Turabian StyleFigueiredo, Elisabete, Catarina Gonçalves, Sónia Duarte, Maria C. Godinho, António Mexia, and Laura Torres. 2021. "Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling" Insects 12, no. 1: 13. https://doi.org/10.3390/insects12010013
APA StyleFigueiredo, E., Gonçalves, C., Duarte, S., Godinho, M. C., Mexia, A., & Torres, L. (2021). Risk Assessment for Tomato Fruitworm in Processing Tomato Crop-Egg Location and Sequential Sampling. Insects, 12(1), 13. https://doi.org/10.3390/insects12010013