Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Infestation in Soybean by Proximal Sensing
Abstract
:Simple Summary
Abstract
1. Introduction
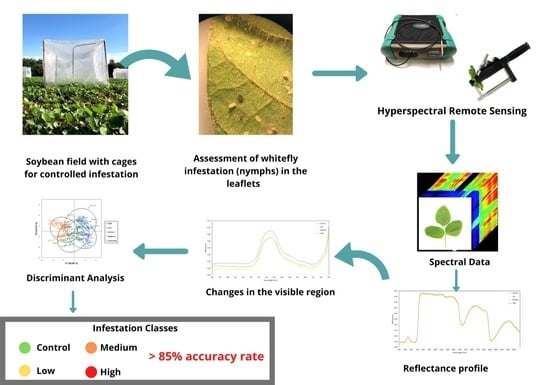
2. Material and Methods
2.1. Local
2.2. Insect Rearing
2.3. Bioassay
2.4. Data Collection
2.5. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture—USDA. World Agricultural Supply and Demand Estimates WASDE-584—11 December 2018. Available online: http://www.usda.gov/oce/commodity/wasde/latest.pdf (accessed on 16 December 2018).
- United States Department of Agriculture—USDA. World Agricultural Production Circular Series WAP 10–20 October 2020. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 29 October 2020).
- Companhia Nacional De Abastecimento—CONAB. Acompanhamento de Safra Brasileira: Grãos, Quarto Levantamento, December 2018. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 18 December 2018).
- Confederação da Agricultura e Pecuária do Brasil—CNA. Panorama do Agro, June 2020. Available online: https://www.cnabrasil.org.br/cna/panorama-do-agro#_ftn1 (accessed on 29 October 2020).
- Boerma, H.R.; Walker, D.R. Discovery and utilization of QTLs for insect resistance in soybean. Genetica 2005, 123, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of insect-damaged vegetable soybeans using hyperspectral transmittance image. J. Food Eng. 2013, 116, 45–49. [Google Scholar] [CrossRef]
- Vieira, S.S.; De Freitas Bueno, R.C.O.; De Freitas Bueno, A.; Boff, M.I.C.; Gobbi, A.L. Different timing of whitefly control and soybean yield. Cienc. Rural 2013, 43, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Dângelo, R.A.C.; Michereff-Filho, M.; Campos, M.R.; Da Silva, P.S.; Guedes, R.N.C. Insecticide resistance and control failure likelihood of the whitefly Bemisia tabaci (MEAM1; B biotype): A Neotropical scenario. Ann. Appl. Biol. 2018, 172, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Stern, V.M.; Smith, R.F.; Van Den Bosch, R.; Hagen, R.S. The integrated control concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Pedigo, L.P.; Higley, L.G. Introduction to pest management and thresholds. In Economic Thresholds for Integrated Pest Management; University of Nebraska Press: Lincoln, NE, USA, 1996; pp. 3–8. [Google Scholar]
- Pedigo, L.P.; Hutchins, S.H.; Higley, L.G. Economic injury levels in theory and practice. Annu. Rev. Entomol. 1986, 31, 341–368. [Google Scholar] [CrossRef]
- Bueno, A.D.F.; Panizzi, A.R.; Corrêa-Ferreira, B.S.; Hoffmann-Campo, C.B.; Sosa-Gómez, D.R.; Gazzoni, D.L.; Roggia, S. Histórico e Evolução do Manejo Integrado de Pragas da Soja no Brasil. In Soja: Manejo Integrado de Insetos e Outros Artrópodes-Praga; Embrapa: Brasília, Brazil, 2012; pp. 37–74. [Google Scholar]
- Flint, M.L. IPM in Practice: Principles and Methods of Integrated Pest Management; UCANR Publications; University of California Agriculture & Natural Resources: Davis, CA, USA, 2012; ISBN 1601077858. [Google Scholar]
- Boyer, W.P.; Dumas, W.A. Plant-shaking methods for soybean insect survey in Arkansas. In SURVEY Methods for Some Economic Insects; USDA: Forrest City, AR, USA, 1969; pp. 92–94. [Google Scholar]
- Shepard, B.M.; Carner, G.R.; Turnipseed, S.G. A Comparison of Three Sampling Methods for Arthropods in Soybean. Environ. Èntomol. 1974, 3, 227–232. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Corrêa, B.S.; Gazzoni, D.L.; De Oliveira, E.B.; Newman, G.G.; Turnipseed, S.G. Insetos da Soja no Brasil; EMBRAPA-CNPSo: Londrina, Brazil, 1977; p. 20, (EMBRAPA-CNPSo. Boletim Técnico, 1). [Google Scholar]
- Iost Filho, F.; Paiva, A.C.; Barros, P.P.S.; Rosalen, D.; Yamamoto, P.T. Assessment of soybean plants’ susceptibility to Bemisia tabaci using remote sensing. In Proceedings of the 2018 ESA, ESC, and ESBC Joint Annual Meeting, Vancouver, BC, Canada, 11–14 November 2018; Entomological Society of America: Annapolis, MD, USA, 2018. [Google Scholar]
- Severtson, D.; Callow, N.; Flower, K.; Neuhaus, A.; Olejnik, M.; Nansen, C. Unmanned aerial vehicle canopy reflectance data detects potassium deficiency and green peach aphid susceptibility in canola. Precis. Agric. 2016, 17, 659–677. [Google Scholar] [CrossRef] [Green Version]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Carrière, Y.; Ellsworth, P.C.; Dutilleul, P.; Ellers-Kirk, C.; Barkley, V.; Antilla, L. A GIS-based approach for areawide pest management: The scales of Lygus hesperus movements to cotton from alfalfa, weeds, and cotton. Entomol. Exp. Appl. 2006, 118, 203–210. [Google Scholar] [CrossRef]
- Backoulou, G.F.; Elliott, N.C.; Giles, K.; Phoofolo, M.; Catana, V. Development of a method using multispectral imagery and spatial pattern metrics to quantify stress to wheat fields caused by Diuraphis noxia. Comput. Electron. Agric. 2011, 75, 64–70. [Google Scholar] [CrossRef]
- Lisboa, I.P.; Damian, J.M.; Cherubin, M.R.; Barros, P.P.S.; Fiorio, P.R.; Cerri, C.C.; Cerri, C.E.P. Prediction of Sugarcane Yield Based on NDVI and Concentration of Leaf-Tissue Nutrients in Fields Managed with Straw Removal. Agronomy 2018, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Fiorio, P.R.; Martins, J.A.; Barros, P.P.S.; Molin, J.P.; Amaral, L.R. Dados espectrais de dossel de cana-de-açúcar para predição do teor relativo de clorofila. In Simpósio Brasileiro De Sensoriamento Remoto; Anais, J.P., Ed.; INPE: São José dos Campos, Brazil, 2015. [Google Scholar]
- Da Silva Junior, C.A.; Nanni, M.R.; Shakir, M.; Teodoro, P.E.; Junior, J.F.O.; Cezar, E.; Gois, G.; Lima, M.; Wojciechoswky, J.C.; Shiratsuchi, L.S. Soybean varieties discrimination using non-imaging hyperspectral sensor. Infrared Phys. Technol. 2018, 88, 338–350. [Google Scholar] [CrossRef]
- Gold, K.M.; Townsend, P.A.; Larson, E.R.; Herrmann, I.; Gevens, A.J. Contact Reflectance Spectroscopy for Rapid, Accurate, and Nondestructive Phytophthora infestans Clonal Lineage Discrimination. Phytopathology 2020, 110, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, S117–S131. [Google Scholar] [CrossRef] [Green Version]
- Nansen, C.; Elliot, N. Remote Sensing and reflectance profiling in entomology. Annu. Rev. Entomol. 2016, 61, 139–158. [Google Scholar] [CrossRef] [Green Version]
- Nansen, C.; Sidumo, A.J.; Capareda, S. Variogram Analysis of Hyperspectral Data to Characterize the Impact of Biotic and Abiotic Stress of Maize Plants and to Estimate Biofuel Potential. Appl. Spectrosc. 2010, 64, 627–636. [Google Scholar] [CrossRef]
- Liu, X.-D.; Sun, Q.-H. Early assessment of the yield loss in rice due to the brown planthopper using a hyperspectral remote sensing method. Int. J. Pest Manag. 2016, 62, 205–213. [Google Scholar] [CrossRef]
- Prasannakumar, N.; Chander, S.; Sahoo, R. Characterization of brown planthopper damage on rice crops through hyperspectral remote sensing under field conditions. Phytoparasitica 2014, 42, 387–395. [Google Scholar] [CrossRef]
- Mirik, M.; Ansley, R.J.; Steddom, K.; Rush, C.M.; Michels, G.J.; Workneh, F.; Cui, S.; Elliott, N.C. High spectral and spatial resolution hyperspectral imagery for quantifying Russian wheat aphid infestation in wheat using the constrained energy minimization classifier. J. Appl. Remote Sens. 2014, 8, 83661. [Google Scholar] [CrossRef] [Green Version]
- Luedeling, E.; Hale, A.; Zhang, M.; Bentley, W.J.; Dharmasri, L.C. Remote sensing of spider mite damage in California peach orchards. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 244–255. [Google Scholar] [CrossRef]
- Chen, T.; Zeng, R.; Guo, W.; Hou, X.; Lan, Y.; Zhang, L. Detection of Stress in Cotton (Gossypium hirsutum L.) Caused by Aphids Using Leaf Level Hyperspectral Measurements. Sensors 2018, 18, 2798. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.E.R.; Rondon, S.I. Detection of potato beetle damage using remote sensing from small unmanned aircraft systems. J. Appl. Remote Sens. 2017, 11, 026013. [Google Scholar] [CrossRef] [Green Version]
- Filho, F.H.I.; Heldens, W.B.; Kong, Z.; De Lange, E.S. Drones: Innovative Technology for Use in Precision Pest Management. J. Econ. Èntomol. 2020, 113, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Alves, T.M.; Moon, R.D.; Macrae, I.V.; Koch, R.L. Optimizing band selection for spectral detection of Aphis glycines Matsumura in soybean. Pest Manag. Sci. 2019, 75, 942–949. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 1st ed.; Bookman: Stockholm, Sweden, 2009; 688p, ISBN 8577805344. [Google Scholar]
- Rencher, A.C. Methods of Multivariate Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 492. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Fereres, A.; Lopes, J.R.S. Settling preferences of the whitefly vector Bemisia tabaci on infected plants varies with virus family and transmission mode. Èntomol. Exp. Appl. 2017, 165, 138–147. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Iowa State University, Agricultural and Home Economics Experiment Station: Ames, IA, USA, 1977. [Google Scholar]
- Department of Biosystems Engineer—LEB. Meteorological Automatic Station Base, March 2019. Available online: http://www.leb.esalq.usp.br/leb/automatica/pagina5.html (accessed on 14 March 2019).
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early detection of Fusarium infection in wheat using hyper-spectral imaging. Comput. Electron. Agric. 2011, 75, 304–312. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: Long Island, NY, USA, 2019; Available online: https://www.xlstat.com (accessed on 19 March 2019).
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Pearson Education India: Noida, India, 2009; 592p, ISBN 9780131889507. [Google Scholar]
- Islam, M.T.; Shunxiang, R. Effect of sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infestation on eggplant (Solanum melongena L.) leaf. J. Pest Sci. 2009, 82, 211–215. [Google Scholar]
- Naranjo, S.E.; Legg, J.P. Biology and ecology of Bemisia tabaci. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 105–107. [Google Scholar]
- Baldin, E.L.L.; Cruz, P.L.; Morando, R.; Silva, I.F.; Bentivenha, J.P.F.; Tozin, L.R.S.; Rodrigues, T.M. Characterization of Antixenosis in Soybean Genotypes to Bemisia tabaci (Hemiptera: Aleyrodidae) Biotype B. J. Econ. Èntomol. 2017, 110, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.E.; Holbrook, N.M.; Zwieniecki, M.A.; Sadok, W.; Sinclair, T.R. Field confirmation of genetic variation in soybean transpiration response to vapor pressure deficit and photosynthetic compensation. Field Crop. Res. 2011, 124, 85–92. [Google Scholar] [CrossRef]





| Actual Class | Assigned Class by Training Model | |||
|---|---|---|---|---|
| High | Low | Control | Medium | |
| High | 17 (73.91%) | 3 | 2 | 1 |
| Low | 2 | 16 (69.57%) | 3 | 2 |
| Control | 1 | 3 | 16 (72.73%) | 2 |
| Medium | 0 | 4 | 0 | 24 (85.71%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, P.P.S.; Schutze, I.X.; Iost Filho, F.H.; Yamamoto, P.T.; Fiorio, P.R.; Demattê, J.A.M. Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Infestation in Soybean by Proximal Sensing. Insects 2021, 12, 47. https://doi.org/10.3390/insects12010047
Barros PPS, Schutze IX, Iost Filho FH, Yamamoto PT, Fiorio PR, Demattê JAM. Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Infestation in Soybean by Proximal Sensing. Insects. 2021; 12(1):47. https://doi.org/10.3390/insects12010047
Chicago/Turabian StyleBarros, Pedro P. S., Inana X. Schutze, Fernando H. Iost Filho, Pedro T. Yamamoto, Peterson R. Fiorio, and José A. M. Demattê. 2021. "Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Infestation in Soybean by Proximal Sensing" Insects 12, no. 1: 47. https://doi.org/10.3390/insects12010047
APA StyleBarros, P. P. S., Schutze, I. X., Iost Filho, F. H., Yamamoto, P. T., Fiorio, P. R., & Demattê, J. A. M. (2021). Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Infestation in Soybean by Proximal Sensing. Insects, 12(1), 47. https://doi.org/10.3390/insects12010047