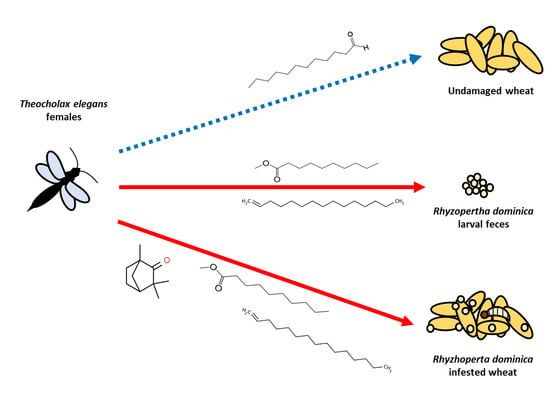
Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Insect Colonies
2.2. General Methods for Bioassays
2.3. No-Choice and Choice Experiments
- Undamaged vs. Infested kernels
- Undamaged vs. Undamaged kernels + Feces
- Infested kernels vs. Undamaged kernels + Feces
- Infested kernels vs. Feces
2.4. Identification of Volatile Organic Compounds (VOCs)
2.5. Data Analysis
3. Results
3.1. Parasitoid Preferences for Different Host-Substrates
3.2. VOCs Identification from Host-Substrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mhlanga, N.; Seidler, E.; Njie, D.; Gallat, S.; Lamb, J.; Morgan, N.; Zorya, S.; Diaz Rios, L. FAO/World Bank Workshop on Reducing Post-Harvest Losses in Grain Supply Chains in Africa; Lessons Learned and Practical Guidelines; FAO: Rome, Italy, 18–19 March 2010; pp. 1–121. Available online: http://www.fao.org/3/a-au092e.pdf (accessed on 14 December 2020).
- Nayak, M.K.; Daglish, G.J. Importance of Stored Product Insects. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–17. ISBN 9783662561256. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential Oils in Stored Product Insect Pest Control. J. Food Qual. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mbata, G.; Warsi, S. Habrobracon hebetor and Pteromalus cerealellae as Tools in Post-Harvest Integrated Pest Management. Insects 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Campolo, O.; Verdone, M.; Laudani, F.; Malacrinò, A.; Chiera, E.; Palmeri, V. Response of four stored products insects to a structural heat treatment in a flour mill. J. Stored Prod. Res. 2013, 54, 54–58. [Google Scholar] [CrossRef]
- Environtal Protection Agency (EPA). Parasitic and predaceous insects used to control insect pests; exemption from a tolerance. Fed. Reg. 1992, 57, 14644–14646. [Google Scholar]
- Schöller, M.; Prozell, S.; Suma, P.; Russo, A. Biological Control of Stored-Product Insects. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 183–209. ISBN 9783662561256. [Google Scholar] [CrossRef]
- Schöller, M.; Flinn, P.W. Parasitoids and predators. In Alternatives to Pesticides in Stored-Product IPM.; Subramanyam, B., Hagstrum, D.W., Eds.; Springer: Boston, MA, USA, 2000; pp. 229–271. [Google Scholar] [CrossRef]
- Amante, M.; Schöller, M.; Suma, P.; Russo, A. Bethylids attacking stored-product pests: An overview. Entomol. Exp. Appl. 2017, 163, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Schöller, M.; Prozell, S.; Al-Kirshi, A.G.; Reichmuth, C. Towards biological control as a major component of integrated pest management in stored product protection. J. Stored Prod. Res. 1997, 33, 81–97. [Google Scholar] [CrossRef]
- Flinn, P.W.; Hagstrum, D.W.; Mcgaughey, W.H. Suppression of beetles in stored wheat by augmentative releases of parasitic wasps. Environ. Entomol. 1996, 25, 505–511. [Google Scholar] [CrossRef]
- Toews, M.D.; Phillips, T.W.; Cuperus, G.W. Effects of wheat cultivar and temperature on suppression of Rhyzopertha dominica (Coleoptera: Bostrichidae) by the parasitoid Theocolax elegans (Hymenoptera: Pteromalidae). Biol. Control 2001, 21, 120–127. [Google Scholar] [CrossRef]
- Van den Assem, J.; Kuenen, D.J. Host finding of Choetospila elegans Westw. (Hym. Chalcid.) a parasite of Sitophilus granarius L. (Coleopt. Curcul.). Entomol. Exp. Appl. 1958, 1, 174–180. [Google Scholar] [CrossRef]
- Dlamini, B.E.; Amornsak, W. Effect of host age on progeny production of Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae) reared on Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Kasetsart J. Nat. Sci. 2014, 48, 587–597. [Google Scholar]
- Wen, B.; Smith, L.; Brower, J.H. Competition between Anisopteromalus calandrae and Choetospila elegans (Hymenoptera: Pteromalidae) at different parasitoid densities on immature maize weevils (Coleoptera: Curculionidae) in corn. Environ. Entomol. 1994, 23, 367–373. [Google Scholar] [CrossRef]
- Wen, B.; Brower, J.H. Competition between Anisopteromalus calandrae and Choetospila elegans (Hymenoptera: Pteromalidae) at different parasitoid densities on immature rice weevils (Coleoptera: Curculionidae) in wheat. Biol. Control 1995, 5, 151–157. [Google Scholar] [CrossRef]
- Flinn, P.W. Temperature Effects on Efficacy of Choetospila elegans. J. Econ. Entomol. 1998, 91, 320–323. [Google Scholar] [CrossRef]
- Helbig, J. Ability of naturally occurring parasitoids to suppress the introduced pest Prostephanus truncatus (Horn) (Coleoptera, Bostrichidae) in traditional maize stores in Togo. J. Stored Prod. Res. 1998, 34, 287–295. [Google Scholar] [CrossRef]
- Flinn, P.W.; Hagstrum, D.W. Augmentative releases of parasitoid wasps in stored wheat reduces insect fragments in flour. J. Stored Prod. Res. 2001, 37, 179–186. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2009, 45, 195–200. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Electrophysiological and Behavioral Responses of Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae) to Cereal Grain Volatiles. Biomed. Res. Int. 2016, 2016, 5460819. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q. Sitophilus zeamais-Induced rice grain volatiles: Attractiveness towards the generalist parasitoid wasp, Theocolax elegans. Pak. J. Zool. 2016, 48, 1817–1824. [Google Scholar]
- Tang, Q. Olfactory responses of Theocolax elegans (Hymenoptera, Pteromalidae) females to volatile signals derived from host habitats. J. Hymenopt. Res. 2016, 49, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Giunti, G.; Benelli, G.; Messing, R.H.; Canale, A. Early adult learning affects host preferences in the tephritid parasitoid Psyttalia concolor (Hymenoptera: Braconidae). J. Pest Sci. 2016, 89, 529–537. [Google Scholar] [CrossRef]
- Steidle, J.L.M.; Lanka, J.; Muller, C.; Ruther, J. The use of general foraging kairomones in a generalist parasitoid. Oikos 2001, 95, 78–86. [Google Scholar] [CrossRef]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef]
- Steidle, J.L.M.; Steppuhn, A.; Ruther, J. Specific Foraging Kairomones Used by a Generalist Parasitoid. J. Chem. Ecol. 2003, 29, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Revadi, S.; Carpita, A.; Giunti, G.; Raspi, A.; Anfora, G.; Canale, A. Behavioral and electrophysiological responses of the parasitic wasp Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae) to Ceratitis capitata-induced fruit volatiles. Biol. Control. 2013, 64, 116–124. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Steam, IL, USA, 1995; ISBN 978-1-932633-21-4. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Acad. Press: New York, NY, USA, 1980; ISBN 0323141056. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley and Sons: New York, NY, USA, 1976. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Giunti, G.; Campolo, O.; Laudani, F.; Algeri, G.M.; Palmeri, V. Olive fruit volatiles route intraspecific interactions and chemotaxis in Bactrocera oleae (Rossi) (Diptera: Tephritidae) females. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 0691000476. [Google Scholar]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the “cry for help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Steidle, J.L.M.; Fischer, A.A.; Gantert, C. Do grains whisper for help? Evidence for herbivore-induced synomones in wheat grains. Entomol. Exp. Appl. 2005, 115, 239–245. [Google Scholar] [CrossRef]
- Benelli, G.; Pacini, N.; Conti, B.; Canale, A. Following a scented beetle: Larval faeces as a key olfactory cue in host location of Stegobium paniceum (Coleoptera: Anobiidae) by Lariophagus distinguendus (Hymenoptera: Pteromalidae). Chemoecology 2013, 23, 129–136. [Google Scholar] [CrossRef]
- Steiner, S.; Steidle, J.L.M.; Ruther, J. Host-associated kairomones used for habitat orientation in the parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2007, 43, 587–593. [Google Scholar] [CrossRef]
- Steidle, J.L.M.; Steppuhn, A.; Reinhard, J. Volatile cues from different host complexes used for host location by the generalist parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). Basic Appl. Ecol. 2001, 2, 45–51. [Google Scholar] [CrossRef]
- Giunti, G.; Palmeri, V.; Algeri, G.M.; Campolo, O. VOC emissions influence intra- and interspecific interactions among stored-product Coleoptera in paddy rice. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, E.M.; Sullivan, B.T.; Anderson, P.; Berisford, C.W.; Birgersson, G. Odor perception in the bark beetle parasitoid Roptrocerus xylophagorum exposed to host associated volatiles. J. Chem. Ecol. 2000, 26, 2507–2525. [Google Scholar] [CrossRef]
- Takabayashi, J.; Takahashi, S. Effects of host fecal pellet and synthetic kairomone on host-searching and postoviposition behavior of Apanteles kariyai, a parasitoid of Pseudaletia separata. Entomol. Exp. Appl. 1989, 52, 221–227. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Heath, R.R.; Proveaux, A.T.; Doolittle, R.E. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 1991, 17, 2235–2251. [Google Scholar] [CrossRef] [Green Version]
- Agelopoulos, N.G.; Dicke, M.; Posthumus, M.A. Role of volatile inforchemicals emitted by feces of larvae in host-searching behavior of parasitoid Cotesia rubecula (Hymenoptera: Braconidae): A behavioral and chemical study. J. Chem. Ecol. 1995, 21, 1789–1811. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Lewis, W.J.; Tumlinson, J.H. Host-specific recognition kairomone for the parasitoid Microplitis croceipes (Cresson). J. Chem. Ecol. 1995, 21, 1697–1708. [Google Scholar] [CrossRef]
- Chiu-Alvarado, P.; Rojas, J.C. Behavioural responses of bethylid parasitoid species of the coffee Berry borer to chemicals cues from host and non-host dust/frass. BioControl 2010, 56, 45–53. [Google Scholar] [CrossRef]
- Steidle, J.L.M.; Fischer, A. Quantity does matter: How feces are used for host stage selection by granary weevil parasitoid Lariophagus distinguendus. J. Chem. Ecol. 2000, 26, 2657–2664. [Google Scholar] [CrossRef]
- Verheggen, F.; Ryne, C.; Olsson, P.O.C.; Arnaud, L.; Lognay, G.; Högberg, H.E.; Persson, D.; Haubruge, E.; Löfstedt, C. Electrophysiological and behavioral activity of secondary metabolites in the confused flour beetle, Tribolium confusum. J. Chem. Ecol. 2007, 33, 525–539. [Google Scholar] [CrossRef]
- Fürstenau, B.; Adler, C.; Schulz, H.; Hilker, M. Host Habitat Volatiles Enhance the Olfactory Response of the Larval Parasitoid Holepyris sylvanidis to Specifically Host-Associated Cues. Chem. Senses 2016, 41, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Banks, H.J.; Sharp, A.K. Insect control with CO2 in a small stack of bagged grain in a plastic film enclosure. Aust. J. Exp. Agric. 1979, 19, 102–107. [Google Scholar] [CrossRef]
- Robacker, D.C.; Weaver, K.M.; Hendry, L.B. Sexual communication and associative learning in the parasitic wasp Itoplectis conquisitor (Say). J. Chem. Ecol. 1976, 2, 39–48. [Google Scholar] [CrossRef]

| Substrates | NS 1 Females (N 4) | NC 2 Females (N) | C 3 Females (N) | Residence Time (s) |
|---|---|---|---|---|
| Infested | 3 | 4 | 26 A | 129.0 ± 15.0 a |
| Undamaged + Feces | 3 | 14 | 16 B | 97.8 ± 20.0 ab |
| Feces | 2 | 12 | 18 B | 73.9 ± 11.7 b |
| Undamaged | 5 | 25 | 5 C | 40.6 ± 12.1 b |
| Trial | Cue A | Cue B | First Choice | Cue A (N 1) | Cue B (N) | Residence Time Cue A (s) | Residence Time Cue B (s) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Infested | Undamaged | Infested | 26 | 2 | * | 170.0 ± 20.6 | 16.5 ± 1.5 | * |
| Undamaged | 2 | 4 | ns 2 | 158.0 ± 26.0 | 143.3 ± 51.3 | ns | |||
| Total | 28 | 6 | * | 169.1 ± 19.1 | 101.0 ± 42.0 | ns | |||
| 2 | Undamaged | Undamaged + Feces | Undamaged | 3 | 2 | ns | 121.0 ± 75.0 | 103.5 ± 67.5 | ns |
| Undamaged + Feces | 0 | 27 | * | 0.0 ± 0.0 | 162.7 ± 19.0 | * | |||
| Total | 3 | 29 | * | 121.0 ± 75.0 | 158.7 ± 18.2 | ns | |||
| 3 | Infested | Undamaged + Feces | Infested | 11 | 7 | ns | 163.8 ± 27.7 | 74.9 ± 16.3 | * |
| Undamaged + Feces | 7 | 19 | ns | 147.3 ± 15 | 170.5 ± 21.9 | ns | |||
| Total | 18 | 26 | ns | 157.4 ± 17.6 | 144.7 ± 18.5 | ns | |||
| 4 | Infested | Feces | Infested | 18 | 0 | * | 179.4 ± 22.1 | 0.0 ± 0.0 | * |
| Feces | 2 | 12 | * | 73.0 ± 9.0 | 213.8 ± 21.9 | * | |||
| Total | 20 | 12 | ns | 168.8 ± 21.2 | 213.8 ± 21.9 | ns |
| Compound | LRI 1 Calculated | LRI Literature | Undamaged (%) | Infested (%) | F 2 | p 3 | Feces (%) |
|---|---|---|---|---|---|---|---|
| Decane | 1000 | 1000 | 33.58 ± 8.21 | 11.84 ± 4.35 | 0.6 | 0.47 | 34.67 ± 9.63 |
| Limonene | 1038 | 1037 | tr 4 | 3.19 ± 3.14 | 0.61 | 0.46 | - |
| Fenchone | 1090 | 1087 | - | 4.78 ± 2.83 | 8.99 | 0.02 * | - |
| Nonanal | 1101 | 1102 | - | 3.6 ± 2.98 | 2.99 | 0.13 | tr |
| 1-Decanol | 1274 | 1274 | 1.19 ± 1.03 | 3.32 ± 1.09 | 3.92 | 0.1 | 5.88 ± 1.36 |
| Tridecane | 1299 | 1300 | 2.2 ± 1.32 | - | 3.00 | 0.13 | - |
| Undecanal | 1310 | 1306 | 0.95 ± 0.66 | 1.63 ± 0.69 | 0.48 | 0.51 | tr |
| Methyl-decanoate | 1331 | 1326 | - | 3.43 ± 1.3 | 7066.36 | <0.001 * | 8.48 ± 2.29 |
| Tetradecane | 1398 | 1400 | tr | - | 1.00 | 0.36 | tr |
| Dodecanal | 1412 | 1409 | 5.9 ± 3.56 | - | 7.99 | 0.03 * | - |
| 1-Pentadecene | 1491 | 1491 | - | 1.67 ± 0.6 | 8.98 | 0.02 * | 0.45 ± 0.25 |
| 1-Hexadecene | 1585 | 1589 | 8.85 ± 8.65 | 0.38 ± 0.38 | 0.001 | 0.98 | - |
| Tetradecanal | 1614 | 1611 | 6.07 ± 2.69 | tr | 1.11 | 0.33 | - |
| 1-Tetradecanol | 1681 | 1676 | 36.67 ± 17.96 | 46.78 ± 10.61 | 1.53 | 0.26 | 47 ± 14.34 |
| Total | 97.04 ± 2.55 | 98.23 ± 1.69 | 96.78 ± 3.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giunti, G.; Campolo, O.; Caccamo, P.; Laudani, F.; Palmeri, V. Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location. Insects 2021, 12, 142. https://doi.org/10.3390/insects12020142
Giunti G, Campolo O, Caccamo P, Laudani F, Palmeri V. Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location. Insects. 2021; 12(2):142. https://doi.org/10.3390/insects12020142
Chicago/Turabian StyleGiunti, Giulia, Orlando Campolo, Pasquale Caccamo, Francesca Laudani, and Vincenzo Palmeri. 2021. "Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location" Insects 12, no. 2: 142. https://doi.org/10.3390/insects12020142
APA StyleGiunti, G., Campolo, O., Caccamo, P., Laudani, F., & Palmeri, V. (2021). Volatile Infochemicals from Rhyzopertha dominica Larvae and Larval Feces Involved in Theocolax elegans Host Habitat Location. Insects, 12(2), 142. https://doi.org/10.3390/insects12020142