Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control
Abstract
:Simple Summary
Abstract
1. Formulations Are Adapted According to Specific Routes of Insect Body Entry, Arthropod Diversity, and Various Environmental Conditions
2. Gas and Vapor Insecticide Application Formulations
2.1. Vaporization and Sublimation
2.1.1. Thermal Vaporization
2.1.2. Cold Vaporization or Sublimation (“Residual Fumigation”)
2.1.3. Preventive vs. Repressive Application Methods of Insecticide Evaporators
2.1.4. Injection/Infusion into and Evaporation Inside Bags (“In-Bag Fumigation”)
2.2. Fumigation—Toxic Gas Release from Solid, Liquid, and Gas Formulations
2.2.1. Fumigants Released from Solid Formulations
2.2.2. Fumigants Released from Liquid or Liquidized Gas Formulations (Compressed in Cylinders, Soaked in Porous Materials)
2.3. Application of Inert Gases as Modified Atmospheres (i.e., Pest Asphyxiation by Hypoxic/Anoxic Atmospheres)
2.3.1. Hermetic Airtight Storage (Bags, Cocoons, Bunkers, Underground Stores and Pits, Under-Sheets)
2.3.2. Controlled Atmospheres in Food Packages, Chambers, Silos, Horizontal Stores, and Flexible Enclosures (Normal/Changed Atmospheric Pressure)
3. Delivery of Insecticides as Liquids (Admixtures, Liquid Baits, Aerosols, Sprays, etc.)
3.1. Grain Protectants—Spray, Drip, Cob-Dip, and Aerosol Treatments (Insecticide Admixture, Top-Dressing)
3.2. Dip and Spray Insecticide Coatings for the Protection of Dried or Smoked Fishes and Animal Skins
3.3. Liquid or Aqueous Baits (Traditional Toxic Baits or “Smart Baits” Based on RNA Interference)
3.4. Insecticide Dipping, Impregnation, and Spraying of Bags and Packages
3.5. Liquid Insecticide Aerosols and Mists: Thermal Fogs and Cold Aerosols (“ULV”, “LV”, “HV”)
3.6. Surface Spray, Brush or Sponge Applications, Leaving Residual Insecticide Deposits
3.6.1. Formulations and Active Compounds Used as Surface-Residual Sprays
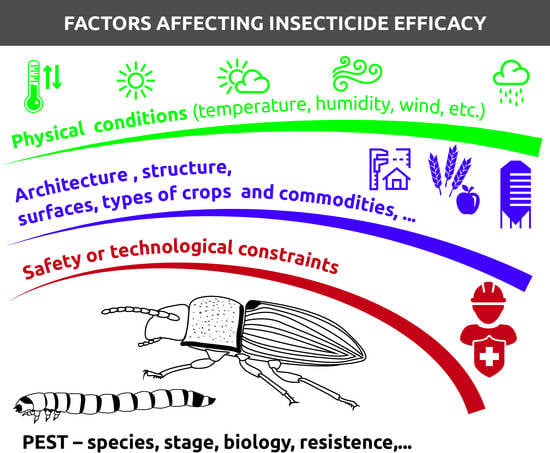
3.6.2. Biotic and Abiotic Factors Influencing the Efficacy of Insecticide Deposits
3.6.3. Equipment and Types of Spray or Brush Applications (Broadcast, Spot, Crack-And-Crevice, Barrier, Direct, and Special Treatments)
4. Insecticide Gel and Foam Application Formulations
4.1. Gel and Paste Baits
4.2. Expandable Insecticide Foams (Baits and Contact Insecticides)
4.3. Acaricide Gels and Coatings (Films, Nets) for Ham Protection
4.4. Gels, Gelatines, Starch Pastes, and Wax Polish Used for Residual Insecticide Pre-Treatment or Co-Treatment
5. Insecticide Delivery in Solid Forms
5.1. Smoke-Generating Formulations (Chemically Activated or Ignition-Activated Pyrotechnic Smoke Generators, Cartridges, Tablets, and Canisters)
5.2. Baits Applied as Solid Dusts, Granules, or Semi-Solid Slurries
5.3. Application of Synthetic or Natural Organic and Inorganic Insecticide Dusts (Structural, Grain Admixture)
5.3.1. Dusts Applied as Surface and Structural Treatments
5.3.2. Dusts Applied as Commodity Admixture Protectants
5.4. Botanical Ash, Dust, Powders, Particles, Leaves, Phyto-Tablets, and Sachets
5.5. Incorporated Insecticides: Seed Dressings, Toxic and Edible Coatings and Films
5.6. Insecticide Incorporated/Impregnated Bags, Packaging Foils and Packages (“Active Packaging”)
5.7. Insecticides Incorporated in Nets and Nettings
6. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Athanassiou, C.G.; Arthur, F.H. Recent Advances in Stored Product Protection; Springer: Berlin, Germany, 2018; p. 273. [Google Scholar]
- Stejskal, V.; Vendl, T.; Li, Z.; Aulicky, R. Efficacy of visual evaluation of insect-damaged kernels of malting barley by Sitophilus granarius from various observation perspectives. J. Stored Prod. Res. 2020, 89, 101711. [Google Scholar] [CrossRef]
- Stejskal, V.; Hubert, J.; Aulicky, R.; Kucerova, Z. Overview of present and past and pest-associated risks in stored food and feed products: European perspective. J. Stored Prod. Res. 2015, 64, 122–132. [Google Scholar] [CrossRef]
- Hubert, J.; Erban, T.; Nesvorna, M.; Stejskal, V. Emerging risk of infestation and contamination of dried fruits by mites in the Czech Republic. Food Addit. Contam. Part A 2011, 28, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol. 2018, 63, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Vendl, T.; Kolar, V.; Li, Z.; Aulicky, R. First population quantification of the infestation of legumes by stored-product bruchids imported in freight containers into Europe. Bull. Insectol. 2020, 73, 233–239. [Google Scholar]
- Nopsa, J.F.H.; Daglish, G.J.; Hagstrum, D.W.; Leslie, J.F.; Phillips, T.W.; Scoglio, C.; Thomas-Sharma, S.; Walter, G.H.; Garrett, K.A. Ecological networks in stored grain: Key postharvest nodes for emerging pests, pathogens, and mycotoxins. Bioscience 2015, 65, 985–1002. [Google Scholar] [CrossRef] [Green Version]
- Fardisi, M.; Gondhalekar, A.D.; Ashbrook, A.R.; Scharf, M.E. Rapid evolutionary responses to insecticide resistance management interventions by the German cockroach (Blattella germanica L.). Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Opit, G.P.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M. Phosphine resistance in Tribolium castaneum and Rhyzopertha dominica from stored wheat in Oklahoma. J. Econ. Entomol. 2012, 105, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [Green Version]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry–The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Zhu, F.; Lavine, L.; O’Neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide resistance and management strategies in urban ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- Capinera, J.L. Encyclopedia of Entomology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ebeling, W.; Pence, R.J. Pesticide formulation. Influence of formulation on effectiveness. J. Agric. Food Chem. 1953, 1, 386–397. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Busvine, J.R. A Critical Review of the Techniques for Testing Insecticides, 2nd ed.; Commonwealth Agricultural Bureaux: Slough, UK, 1971; p. 345. [Google Scholar]
- Wardle, R.A. The Problems of Applied Entomology; Manchester University Press: Manchester, UK, 1929; p. 587. [Google Scholar]
- Gerolt, P. Insecticides—their route of entry, mechanism of transport and mode of action. Biol. Rev. Camb. Philos. Soc. 1983, 58, 233–274. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Lavidis, N.A.; Mensah, R.K.; Choy, P.T.; Walter, G.H. The toxicological effects of petroleum spray oils on insects–Evidence for an alternative mode of action and possible new control options. Food Chem. Toxicol. 2008, 46, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.E.; Green, R.A. On the penetration of insecticides through the insect cuticle. J. Exp. Biol. 1945, 22, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Busvine, J.R. Arthropod Vectors of Disease. In Studies in Biology; No. 55; Edward Arnold: London, UK, 1975; p. 67. [Google Scholar]
- Hagstrum, D.W.; Phillips, T.W. Evolution of stored-product entomology: Protecting the world food supply. Annu. Rev. Entomol. 2017, 62, 379–397. [Google Scholar] [CrossRef]
- Maier, D.E. Advances in Postharvest Management of Cereals and Grains; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; p. 300. [Google Scholar]
- Stejskal, V.; Vendl, T.; Li, Z.; Aulicky, R. Minimal thermal requirements for development and activity of stored product and food industry pests (Acari, Coleoptera, Lepidoptera, Psocoptera, Diptera and Blattodea): A review. Insects 2019, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Jian, F. Influences of stored product insect movements on integrated pest management decisions. Insects 2019, 10, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, J.W. Pests of Stored Products; The Rentokil Library, Hutchinson: London, UK, 1966. [Google Scholar]
- Snell, E.J. Future trends in pesticide applications. In Proceedings of the 3rd International Conference Urban Pest, Prague, Czech Republic, 19–22 July 1999; pp. 35–41. [Google Scholar]
- Robinson, W.H. The Service Technician’s Application and Equipment Manual: A Practical Guide for Pest Control Professionals; Lupo, L.J., Ed.; PCT—Pest Control Technology: Valley View, OH, USA, 2015; p. 128. [Google Scholar]
- Murdock, L.L.; Seck, D.; Ntoukam, G.; Kitch, L.; Shade, R.E. Preservation of cowpea grain in sub-Saharan Africa—Bean/Cowpea CRSP contributions. Field Crops Res. 2003, 82, 169–178. [Google Scholar] [CrossRef]
- Stathers, T.; Holcroft, D.K.L.; Mvumi, B.; English, A.; Omotilewa, O.; Kocher, M.; Ault, J.; Torero, M. A scoping review of interventions for crop postharvest loss reduction in sub-Saharan Africa and South Asia. Nat. Sustain. 2020, 3, 821–835. [Google Scholar] [CrossRef]
- Sparks, T.C.; Wessels, F.J.; Lorsbach, B.A.; Nugent, B.M.; Watson, G.B. The new age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic. Biochem. Physiol. 2019, 161, 12–22. [Google Scholar] [CrossRef]
- Freeman, J.A. Pest infestation control in breweries and maltings. J. Inst. Brew. 1951, 57, 326–337. [Google Scholar] [CrossRef]
- Hill, D.S. Pests of Stored Foodstuffs and Their Control; Kluwer Academic Publishers: Boston, MA, USA, 2002. [Google Scholar]
- Peckman, P.S.; Arthur, F.H. Insecticide space treatments in food plants. In Insect Management for Food Storage and Processing; Heaps, J., Ed.; AACC: Minneapolis, MN, USA, 2006; pp. 175–182. [Google Scholar]
- Arthur, F.H. Structural Pest Management for Stored Product Insects. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Chapter 4; pp. 65–81. [Google Scholar]
- Matthews, G.; Bateman, R.; Miller, P. Pesticide Application Methods, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Golob, P.; Farrell, G.; Orchard, J.E. Crop Post-harvest: Principles and practice. In Crop Post-Harvest: Science and Technology; Golob, P., Farrell, G., Orchard, J.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Rust, M.K.; Owens, J.M.; Reierson, D.A. Understanding and Controlling the German Cockroach; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Rust, M.K. The Changing Role of Insecticides in Structural Pest Control. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 257–270. [Google Scholar]
- Daglish, G.J.; Nayak, M.K.; Arthur, F.H.; Athanassiou, C.G. Insect Pest Management in Stored Grain. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Robinson, W. Urban Insects and Arachnids: A Handbook of Urban Entomology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: Saint Paul, MN, USA, 2009; p. 509. [Google Scholar]
- Sutar, S.A.; Thirumdas, R.; Chaudhari, B.B.; Deshmukh, R.K.; Annapure, U.A. Effect of cold plasma on insect infestation and keeping quality of stored wheat flour. J. Stored Prod. Res. 2021, 92, 101774. [Google Scholar] [CrossRef]
- Plimmer, J. Pesticides for Stored Products. In Biodegradation of Pesticides; Mastumura, F., Krishma, C., Eds.; Plenum Press: New York, NY, USA, 1982; pp. 239–255. [Google Scholar]
- Thoms, E.M.; Busacca, J.D. Fumigants. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 150–156. [Google Scholar]
- Bond, E.J. 1984: Manual of Fumigation for Insect Control; FAO: Rome, Italy, 1984; Volume 54, p. 432. [Google Scholar]
- Baur, F.J. Insect Management for Food Storage and Processing; ACCC International: St. Paul, MN, USA, 1984; p. 384. [Google Scholar]
- Stejskal, V.; Kocourek, V.; Aulicky, R.; Hajslova, J. Insecticide Aerosols in Storage IPM: Biological Efficacy and Residues in Air and Food. In Proceedings of the 11th International Working Conference on Stored Product Prot., Chiang Mai, Thailand, 24–28 November 2014; Arthur, F.H., Kengkanpanich, R., Chayaprasert, W., Suthisut, D., Eds.; Julius-Kühn-Archiv: Berlin, Germany, 2014; p. 903. [Google Scholar]
- Roark, R.C.; Nelson, O.A. Maximum weights of various fumigants which can exist in vapor form in a 1,000 cubic foot fumigating chamber. J. Econ. Entomol. 1929, 22, 381–387. [Google Scholar] [CrossRef]
- Jian, F.; Jayas, D.S. Engineering considerations for creating uniform distribution of applied gas during controlled atmospheres and fumigation. In Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products, New Dehli, India, 6–11 November 2016; pp. 1–11. [Google Scholar]
- Jiang, X.; Huang, L.F.; Zheng, S.H.; Chen, S.L. Sulfur fumigation, a better or worse choice in preservation of traditional Chinese medicine? Phytomedicine 2013, 20, 97–105. [Google Scholar] [CrossRef]
- Monro, H.A.U. Manual of Fumigation for Insect Control, 2nd ed.; St. Paul’s Press, Malta for United Nations FAO: Rome, Italy, 1969; p. 381. [Google Scholar]
- Bell, C.H. Fumigation in the 21st century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V.; Frydova, B.; Athanassiou, C.G. Susceptibility of two strains of the confused flour beetle (Coleoptera: Tenebrionidae) following phosphine structural mill fumigation: Effects of concentration, temperature, and flour deposits. J. Econ. Entomol. 2015, 108, 2823–2830. [Google Scholar] [CrossRef]
- Mahroof, R.M.; Amoah, B.A.; Wrighton, J. Efficacy of ozone against the life stages of Oryzaephilus mercator (Coleoptera: Silvanidae). J. Econ. Entomol. 2018, 111, 470–481. [Google Scholar] [CrossRef]
- Navarro, S.; Navarro, H. Advances in insect pest management in postharvest storage of cereals: Use of controlled atmosphere and temperature control. In Advances in Postharvest Management of Cereals and Grains; Maier, D.E., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2020; p. 478. [Google Scholar]
- Rajendran, S. Insect pest management in stored products. Outlooks Pest. Manag. 2020, 31, 24–35. [Google Scholar] [CrossRef]
- Liu, Y.B. Comparison of efficacy of nitric oxide fumigation under nitrogen and carbon dioxide atmospheres in controlling granary weevil (Sitophilus granaries) and confused flour beetle (Tribolium confusum). J. Stored Prod. Res. 2020, 88, 101672. [Google Scholar] [CrossRef]
- Phillips, T.; Thoms, E.; DeMark, J.; Walse, S. Fumigation. In Stored Product Protection, Circular S156; Hagstrum, D.H., Phillips, T.W., Cuperus, G.W., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 157–178. [Google Scholar]
- Arthur, F.H.; Johnson, J.A.; Neven, L.G.; Hallman, G.J.; Follett, P.A. Insect pest management in postharvest ecosystems in the United States of America. Outlooks Pest. Manag. 2009, 20, 279–284. [Google Scholar] [CrossRef]
- Douda, O.; Stejskal, V.; Manasova, M.; Zouhar, M.; Hnatek, J. Inexpensive screening method to validate the efficacy of ethanedinitrile fumigant on the forest invasive nematode pest Bursaphelenchus xylophilus. Sustainability 2020, 12, 4765. [Google Scholar] [CrossRef]
- Douda, O.; Manasova, M.; Zouhar, M.; Hnatek, J.; Stejskal, V. Field validation of the effect of soil fumigation of ethanedinitrile (EDN) on the mortality of Meloidogyne hapla and carrot yield parameters. Agronomy 2021, 11, 208. [Google Scholar] [CrossRef]
- Armstrong, J.W.; Brash, D.W.; Waddell, B.C. Comprehensive literature review of fumigants and disinfestation strategies, methods and techniques pertinent to potential use as quarantine treatments for New Zealand export logs. Plant Food Res. SPTS 2014, 10678, 1–184. [Google Scholar]
- Stejskal, V.; Douda, O.; Zouhar, M.; Manasova, M.; Dlouhy, M.; Simbera, J.; Aulicky, R. Wood penetration ability of hydrogen cyanide and its efficacy for fumigation of Anoplophora glabripennis, Hylotrupes bajulus (Coleoptera), and Bursaphelenchus xylophilus (Nematoda). Int. Biodeter. Biodegr. 2014, 86, 189–195. [Google Scholar] [CrossRef]
- Neven, L.G. Postharvest management of insects in horticultural products by conventional and organic means, primarily for quarantine purposes. Stewart Postharvest Rev. 2010, 6, 1–11. [Google Scholar]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Ajesh, G.; Jayaprakas, C.A.; Krishnan, J.U.; Rajeswari, L.S. Fumigant activity of insecticidal principles isolated from cassava (Manihot esculenta Crantz) against Tribolium castaneum and Rhyzopertha dominica. J. Entomol. Zool. Stud. 2018, 6, 220–225. [Google Scholar]
- Mora, C.A.; Halter, J.G.; Adler, C.; Hund, A.; Anders, H.; Yu, K.; Stark, W.J. Application of the Prunus spp. cyanide seed defense system onto wheat: Reduced insect feeding and field growth tests. J. Agric. Food Chem. 2016, 64, 3501–3507. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Follett, P.A.; Rivera-Leong, K.; Myers, R.Y. Rice weevil response to basil oil fumigation. J. Asia Pac. Entomol. 2013, 17, 119–121. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.-B.; Feng, Y.; Zhang, A. Methyl benzoate fumigation for control of post-harvest pests and its effects on apple quality. J. Appl. Entomol. 2020, 144, 191–200. [Google Scholar] [CrossRef]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A. Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored-product insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, T.; Feng, G.; Feng, Y.; Zhang, A.; Grodowitz, M.J. Insecticidal activity of methyl benzoate analogs against red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2019, 112, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafiz, M.M.; Hassan, E.; Acharya, R.; Shim, J.K.; Lee, K.Y. Methyl benzoate is superior to other natural fumigants for controlling the Indian meal moth (Plodia interpunctella). Insects 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.R.; Zhang, A.; Feldlaufer, M.F. Fumigation activities of ethyl benzoate and its derivatives against the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2020, 57, 187–191. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, A. A floral fragrance methyl benzoate is an efficient green pesticide. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Panagiotakopulu, E.; Buckland, P.C.; Day, P.M.; Sarpaki, A.A.; Doumas, C. Natural insecticides and insect repellents in antiquity: A review of the evidence. J. Archaeol. Sci. 1995, 22, 705–710. [Google Scholar] [CrossRef]
- Hartzell, A. Naphthalene fumigation of greenhouses. J. Econ. Entomol. 1926, 19, 780–786. [Google Scholar] [CrossRef]
- Bourcart, E. Insecticides, Fungicides and Weedkillers: A Practical Manual on the Diseases of Plants and Their Remedies for the Use of Manufacturing Chemists, Agriculturists, Arborculturists, and Horticulturists, 1st ed.; Scott, Greenwood and Son: London, UK, 1913; p. 431. [Google Scholar]
- Gnadinger, C.B. Pyrethrum Flowers, 2nd ed.; McLaughlin Gormley King Co.: Minneapolis, MN, USA, 1936; pp. 281–284. [Google Scholar]
- Frear, D.E.H. Chemistry of Insecticides, Fungicides and Herbicides; D. Van Nostrand Company, Inc.: New York, NY, USA, 1942; p. 300. [Google Scholar]
- Spear, P.J. Laboratory Tests with Insecticides Dispersed from the American Aerovap. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 1950. [Google Scholar]
- Siakotos, A.N. Contamination of Food and Air by Lindane Vapor. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 1954. [Google Scholar]
- Stammers, F.M.; Whitfield, F.G. The toxicity of DDT to man and animals. Bull. Entomol. Res. 1947, 38, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.J. Continuous Vaporization of Insecticides. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 1952. [Google Scholar]
- Braid, P.E.; LeBoeuf, J. Determination of trace amounts of lindane in air by infrared spectrophotometry. Anal.Chem. 1957, 29, 1625–1627. [Google Scholar] [CrossRef]
- Spear, P.J.; Sweetman, H.L. Continuous vaporization of insecticides with special reference to DDT. J. Econ. Entomol. 1952, 45, 869–873. [Google Scholar] [CrossRef]
- Diptyanusa, A.; Satoto, T.B.T.; Hadianto, T. Trial of neem oil (Azadirachta indica) as basic compound of electric liquid vaporizer against Aedes aegypti mortality. YARSI Med. J. 2017, 25, 23–32. [Google Scholar]
- Valecha, N.; Ansari, M.A.; Prabhu, S.; Razdan, R.K. Preliminary evaluation of safety aspects of neem oil in kerosene lamp. Indian J. Malariol. 1996, 33, 139–143. [Google Scholar] [PubMed]
- Ansari, M.A.; Razdan, R.K. Operational feasibility of malaria control by burning neem oil in kerosene lamp in Beel Akbarpur village, District Ghaziabad, India. Indian J. Malariol. 1996, 33, 81–87. [Google Scholar]
- Busvine, J.R. Insects and Hygiene: The Biology and Control of Insect Pests of Medical and Domestic Importance in Britain, 2nd ed.; Methuen & Co.: London, UK, 1966; p. 467. [Google Scholar]
- Wright, D. Mercury as a control for stored grain pests. Bull. Entomol. Res. 1944, 35, 143–160. [Google Scholar] [CrossRef]
- Gough, H.C. Toxicity of mercury vapour to insects. Nature 1938, 141, 922–923. [Google Scholar] [CrossRef]
- Larson, A.O. Metallic mercury as an insecticide. J. Econ. Entomol. 1922, 15, 391–395. [Google Scholar] [CrossRef]
- Herrick, G.W.; Griswold, G.H. Naphthalene as a fumigant for the immature stages of clothes moths and carpet beetles. J. Econ. Entomol. 1933, 26, 446–451. [Google Scholar] [CrossRef]
- Batth, S.S. Influence of temperature on the effectiveness of paradichlorobenzene fumigation of black carpet beetle larvae. J. Econ. Entomol. 1969, 62, 747–748. [Google Scholar] [CrossRef]
- Ryckman, R.E. Vapona for the control of museum pests. J. Med. Entomol. 1969, 6, 98. [Google Scholar] [CrossRef]
- Batth, S.S. Influence of temperature on the effectiveness of paradichlorobenzene fumigation of webbing clothes moth larvae. J. Econ. Entomol. 1971, 64, 989–990. [Google Scholar] [CrossRef]
- Jay, E.G.; Gillenwater, H.B.; Harein, P.K. The toxicity of several dichlorvos (DDVP) and nailed formulations to the adult confused flour beetle. J. Econ. Entomol. 1964, 57, 415–416. [Google Scholar] [CrossRef]
- Harein, P.K.; Gillenwater, H.B.; Eason, G. Dichlorvos space treatment for protection of packaged flour against insect infestation. J. Stored Prod. Res. 1971, 7, 57–62. [Google Scholar] [CrossRef]
- Harein, P.K.; Gillenwater, H.B.; Jay, E.G. Dichlorvos: Methods of dispensing, estimates of concentration in air, toxicity to stored-product insects. J. Econ. Entomol. 1970, 63, 1263–1268. [Google Scholar] [CrossRef]
- Lehnert, M.P.; Pereira, R.M.; Koehler, P.G.; Walker, W.; Lehnert, M.S. Control of Cimex lectularius using heat combined with dichlorvos resin strips. Med. Vet. Entomol. 2011, 25, 460–464. [Google Scholar] [CrossRef]
- Ujihara, K.; Sugano, M.; Nakada, K.; Iwakura, K.; Nishihara, K.; Katoh, H. Discovery and development of profluthrin (Fairytale), a new active ingredient of moth proofer. Sumitomo Kagaku 2010, 2, 13–23. [Google Scholar]
- Abdel-Mohdy, F.A.; Fouda, M.M.; Rehan, M.F.; Aly, A.S. Repellency of controlled-release treated cotton fabrics based on cypermethrin and prallethrin. Carbohydr. Polym. 2008, 73, 92–97. [Google Scholar] [CrossRef]
- Ansari, M.A.; Sharma, V.P.; Razdan, R.K. Esbiothrin-impregnated ropes as mosquito repellent. Indian J. Malariol. 1992, 29, 203–210. [Google Scholar]
- Bullington, S.W.; Pienkowski, R.L. Dichlorvos and plastic covers affect insects infesting stored cocoa beans in dockside warehouses. J. Econ. Entomol. 1993, 86, 1151–1156. [Google Scholar] [CrossRef]
- Peters, L. EC78-1534 Insect Prevention and Control in Farm Stored Grain; Historical Materials from University of Nebraska-Lincoln Extension; University of Nebraska: Lincoln, USA, 1978. [Google Scholar]
- Gillenwater, G.B.; Harein, P.K.; Loy, E.W.; Thompson, J.F.; Laudani, H.; Gerald Eason, G. Dichlorvos applied as a vapor in a warehouse containing packaged foods. J. Stored Prod. Res. 1971, 7, 45–56. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Verfahren zur Untersuchung der Wirkungs abhängigkeit bei Dichlorvos (DDVP-) abgebenden Strips auf die Mortalität vorratsschädlicher Insekten am Beispiel von Plodia interpunctella (Dörrobstmotte). Nachr. Dtsch. Pflanzenschutzd. 1992, 44, 152–156. [Google Scholar]
- Boina, D.R.; Subramanyam, B. Insect management with aerosols in food processing facilities. In Insecticides: Advances in Integrated Pest Management; Perveen, F.K., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 195–212. [Google Scholar]
- Boles, H.P.; Bry, R.E.; Mc Donald, L.L. Dichlorvos vapours: Toxicity to larvae of the furniture carpet beetle. J. Econ. Entomol. 1974, 67, 308–309. [Google Scholar] [CrossRef]
- Endrödy-Younga, S.; Baunok, I. Efficiency and health hazards tests on Vapona used in insect collections. Entomol. Gen. 1984, 10, 47–51. [Google Scholar] [CrossRef]
- Linnie, M.J.; Keatinge, M.J. Pest control in museums: Toxicity of para-dichlorobenzene, ‘Vapona’™, and naphthalene against all stages in the life-cycle of museum pests, Dermestes maculatus Degeer, and Anthrenus verbasci (L.) (Coleoptera: Dermestidae). Int. Biodeterior. Biodegradation 2000, 45, 1–13. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, S.N. Fumigant toxicity of essential oils against four major storage insect pests. Indian J. Entomol. 2017, 79, 156–159. [Google Scholar] [CrossRef]
- Bengston, M. Timed daily emission of dichlorvos for control of Ephestia cautella (Walker) infesting stored wheat. J. Stored Prod. Res. 1976, 12, 157–164. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V.; Opit, G. Short-exposure biological activity of dichlorvos insecticide strips on coleopteran storage pests under two evaporation regimes: Can slow-release dichlorvos formulations replace aerosols? Pak. J. Zool. 2019, 51, 475–482. [Google Scholar] [CrossRef]
- Taylor, R.W.D. Fumigation of individual sacks of grain using methallyl chloride for control of maize weevil. Int. Pest Control. 1975, 17, 4–8. [Google Scholar]
- Green, A.A.; Wilkin, D.R. The control of insects in bagged grain by the injection of dichlorvos. J. Stored Prod. Res. 1969, 5, 11–19. [Google Scholar] [CrossRef]
- Webley, D.J.; Hams, A.H. A comparison of fumigants for in-bag fumigation. Trop. Stored Prod. Inf. 1977, 33, 9–17. [Google Scholar]
- Peirrot, R.; Ducom, P. Efficacy of carbon tetrachloride in sealed sacks compared with contact insecticides. In Proceedings of the GASCA Seminar on Appropriate Use of Pesticides for the Control of Stored Product Pests in Developing Countries; Central Science Laboratory (CSL): Slough, UK, 1981; pp. 149–152. [Google Scholar]
- Knight, K.L. Fumigation of sacked grain with chloropicrin. J. Econ. Entomol. 1940, 33, 536–539. [Google Scholar] [CrossRef]
- Tola, B.Y.; Muleta, D.O.; Werner, C.H. Selection, characterization and identification of smokes from different biomass materials as a medium for modifying the atmosphere for stored grain. J. Stored Prod. Postharvest Res. 2018, 9, 104–114. [Google Scholar]
- Hole, B.D.; Bell, C.H.; Mills, K.A.; Goodship, G. The toxicity of phosphine to all developmental stages of thirteen species of stored product beetles. J. Stored Prod. Res. 1976, 12, 235–244. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Phillips, T.W.; Arthur, F.H.; Aikins, M.J.; Agrafioti, P.; Hartzer, K.L. Efficacy of phosphine fumigation for different life stages of Trogoderma inclusum and Dermestes maculatus (Coleoptera: Dermestidae). J. Stored Prod. Res. 2020, 86, 101556. [Google Scholar] [CrossRef]
- Banks, H.J. Behaviour of gases in grain storages. In Fumigation and Controlled Atmosphere Storage of Grain, Proceedings of the International Conference, Singapore, 14–18 February 1989; Champ, B.R., Highley, E., Banks, H.J., Eds.; ACIAR Proceedings No. 25; PageCraft Publishing Pty Ltd.: Canberra, Australia, 1989; pp. 96–107. [Google Scholar]
- Berck, B. Analysis of fumigants and fumigant residues. J. Chromatogr. Sci. 1975, 13, 256–267. [Google Scholar] [CrossRef]
- Daglish, G.J. Opportunities and barriers to the adoption of potential new grain protectants and fumigants. In Proceedings of the 9th International Working Confonference on Stored Product Protection, Campinas, Brazil, 15–18 October 2006; pp. 209–216. [Google Scholar]
- Anonymous. Fumigation with the Liquid Fumigants Carbon Tetrachloride, Ethylene Dichloride and Ethylene Dibromide—Precautionary Measures; Ministry of Agriculture, Fisheries and Food: London, UK, 1966; p. 9.
- Dean, G.A.; Cotton, R.T.; Wagner, G.B. Flour-Mill Insects and Their Control; U.S. Department of Agriculture: Washington, DC, USA, 1936; p. 360.
- Walkden, H.H.; Schwitzgebel, R.B. Evaluations of Fumigations for Control of Insects Attacking Wheat and Corn in Steel Bins; U.S. Department of Agriculture Technical Bulletin No. 1045: Washington, DC, USA, 1951; p. 20.
- Ramey, C.A.; Schafer, E.W., Jr. The evolution of APHIS two gas cartridges. Proc. Vertebr. Pest Conf. 1996, 17, 219–224. [Google Scholar]
- Lemay, A.; Hall, T. The use of carbon monoxide in wildlife damage management. In Human Health and Ecological Risk Assessment for the Use of Wildlife Damage Management Methods by USDA-APHIS-Wildlife Services; Hall, T., Algeo, T., Green, M., Lemay, A., Wang-Cahill, F., Warren, J., Wimberly, R., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2017; pp. 1–41. [Google Scholar]
- Chittenden, F.H. Control of the Mediterranean Flour Moth by Hydrocyanic-Acid Gas Fumigation; U.S. Department of Agriculture, Bureau of Entomology: Washington, DC, USA, 1910; p. 22.
- Lindgren, D.L.; Vincent, L.E.; Strong, R.G. Studies on hydrogen phosphide as a fumigant. J. Econ. Entomol. 1958, 51, 900–903. [Google Scholar] [CrossRef]
- Reichmuth, C. Phosphine fumigation-new aspects in stored products protection. Gesunde Pflanz. 1975, 37, 417–420. [Google Scholar]
- Ryan, R.F.; Nicolson, J. UltraPhos: High purity phosphine—Revisited. In Proceedings of the 11th International Working Conference on Stored Product Protection, Chaing Mai, Thailand, 24–28 November 2014; Arthur, F.H., Kengkanpanich, R., Chayaprasert, W., Suthisut, D., Eds.; Julius-Kühn-Archiv: Berlin, Germany, 2014; pp. 510–522. [Google Scholar]
- Anonymous. Cyanamid, Patent Appeal No. 2315-2315—California Cyanide Co. v. Am. Cyanamid Co., Patent Appeal No-40 F.2d 1003, 17 C.C.P.A. 1198 (C.C.P.A. 1930). 1930. Available online: https://case-law.vlex.com/vid/40-f-2d-1003-602840598 (accessed on 24 June 2021).
- Reichmuth, C.h. Silozellenbegasung mit Phosphorwasserstoff aus Beutelrollen (Bag Blankets) [Silo bin fumigation with phosphine from bag blankets]. Muhle Mischfuttertech. 1983, 120, 503–504. [Google Scholar]
- Dieterich, W.H.; Mayr, G.; Hild, K.; Sullivan, J.B.; Murphy, J. Hydrogen phosphide as a fumigant for foods, feeds and processed food products. Residue Rev. 1967, 19, 135–149. [Google Scholar] [PubMed]
- Reichmuth, C. Uptake of phosphine by stored-product pest insects during fumigation. In Proceedings of the 6th International Working Conference on Stored-product Protection, Canderra, Australia, 17–23 April 1994; pp. 157–162. [Google Scholar]
- Chadda, I.C. Fumigation with phosphine-a perspective. Indian J. Entomol. 2016, 78, 39–44. [Google Scholar] [CrossRef]
- Agrafioti, P.; Sotiroudas, V.; Kaloudis, E.; Bantas, S.; Athanassiou, C.G. Real time monitoring of phosphine and insect mortality in different storage facilities. J. Stored Prod. Res. 2020, 89, 101726. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V. Efficacy and limitations of phosphine “spot-fumigation” against five Coleoptera species of stored product pests in wheat in a grain store—Short note. Plant Prot. Sci. 2015, 51, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Feja, F.H.; Reichmuth, C.U.S. Patent No. 6, 315,965; Patent and Trademark Office: Washington, DC, USA, 2001. [Google Scholar]
- Ryan, R.F.; Shore, W.; Newman, C. Phosphine generator trial using external air dilution. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Julius-Kühn-Archiv: Berlin, Germany, 2010; pp. 430–432. [Google Scholar]
- Liu, Y.B. Low-Temperature fumigation of harvested lettuce using a phosphine generator. J. Econ. Entomol. 2018, 111, 1171–1176. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Trostanetsky, A.; Yasinov, G.; Menasherov, M.; Hazan, T. Improvement of phosphine fumigation by the use of Speedbox. Julius-Kühn-Archiv 2010, 425, 377–380. [Google Scholar]
- Waterford, C.J.; Asher, P.P. Trials of two phosphine generators based on a new formulation of aluminium phosphide. In Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; Executive Printing Services: Clovis, CA, USA, 2001; pp. 327–333. [Google Scholar]
- Formato, A.; Naviglio, D.; Pucillo, D.P.; Nota, G. Improved fumigation process for stored foodstuffs by using phosphine in sealed chambers. J. Agric. Food Chem. 2012, 60, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.L.; Glasgow, R.D. DDT thermal aerosol fogs to control clothes moths in a wool storage warehouse. J. Econ. Entomol. 1946, 39, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Aulicky, R.; Stejskal, V.; Dlouhy, M.; Liskova, J. Validation of hydrogen cyanide fumigation in flourmills to control the confused flour beetle. Czech J. Food Sci. 2015, 33, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, V.; Adler, C. Fumigation and Controlled Atmospheres; (Fumigace a řízené atmosféry); Sdružení DDD: Prague, Czech Republic, 1997; p. 128. [Google Scholar]
- Loucks, M.F. Composition of Grain Fumigants. J. AOAC Int. 1965, 48, 576–579. [Google Scholar] [CrossRef]
- Quinlan, J.K.; McGaughey, W.H. Fumigation of empty grain drying bins with chloropicrin, phosphine, and liquid fumigant mixtures. J. Econ. Entomol. 1983, 76, 184–187. [Google Scholar] [CrossRef]
- Ren, Y.; Lee, B.; Mahon, D.; Xin, N.; Head, M.; Reid, R. Fumigation of wheat using liquid ethyl formate plus methyl isothiocyanate in 50-tonne farm bins. J. Econ. Entomol. 2008, 101, 623–630. [Google Scholar] [CrossRef]
- Li, Y.; Agarwal, M.; Cao, Y.; Li, F.; Ren, Y. Field trials using ethyl formate as grain surface and empty silo treatments. In Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products, New Delhi, India, 6–11 November 2016; Navarro, S., Jayas, D.S., Alagusundaram, K., Eds.; CAF Permanent Committee Secretariat: Winnipeg, MB, Canada, 2016; pp. 244–251. [Google Scholar]
- Bridgeman, B.; Ryan, R.; Gock, D.; Collins, P. High dose phosphine fumigation using on-site mixing. In Proceedings of the International Conference Controlled Atmosphere and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; Donahaye, E.J., Navarro, S., Leesch, J.G., Eds.; Executive Printing Services: Clovis, CA, USA, 2001; pp. 379–389. [Google Scholar]
- Ryan, R.F. Gaseous Phosphine—A revitalised fumigant. In Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products, Nicosia, Cyprus, 21–26 April 1996; Donahaye, E.J., Navarro, S., Varnava, A., Eds.; Printco Ltd.: Nicosia, Cyprus, 1997; pp. 305–310. [Google Scholar]
- Ryan, R.F.; Latif, S. Fumigant System. US Patent 4 1989, 889,708, 26 December 1989. [Google Scholar]
- Ryan, R.F.; Shore, W.P. Pre-mix and on-site mixing of fumigants. Julius-Kühn-Archiv 2010, 425, 419. [Google Scholar]
- Ryan, R.; Krishna, H.; Bishop, S.R.; Fontinha, M.; Grant, N.; van Epenhuijsen, C.W.; Page, B.; Zhang, Z.; Brash, D.; Mitcham, E.J. Disinfestation and quarantine fumigation. In Proceedings of the Australian Postharvest Horticulture Conference, Brisbane, Australia, 1–3 October 2003; Queensland Government, Department of Primary Industries: Brisbane City, QLD, Australia, 2003; pp. 102–104. [Google Scholar]
- Mueller, D.K. Patent on Phosphine, Carbon Dioxide and Heat against Stored Product Pest Insects; Quoted in Mueller, K.D. Stored Product Protection A Period of Transition; Insects Limited, Inc.: Indianapolis, IN, USA, 1998; p. 352. [Google Scholar]
- Adler, C.; Corinth, H.G.; Reichmuth, C. Modified atmospheres. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D.W., Eds.; Kluwer: Boston, MA, USA, 2000; pp. 105–146. [Google Scholar]
- Dendy, A. Report on the effect of air-tight storage upon grain insects. Part I. Rep. Grain Pests (War) Comm. 1918, 1, 6–24. [Google Scholar]
- Dendy, A.; Elkington, H. Report on the effect of airtight storage upon grain insects. Part III. Rep. Grain Pests (War) Comm. 1920, 6, 51. [Google Scholar]
- Froggatt, W.W. Fumigation maize with carbon dioxide. Agric. Gaz. 1921, 32, 472. [Google Scholar]
- Winterbottom, D.C. Weevil in Wheat Storage of Grain in Bags: A Record of Australian Experience during the War Period. (1915–1919); Government: Adelaide, Australia, 1920; p. 122.
- Navarro, S. Modified atmospheres for the control of stored-product insects and mites. In Insect Management for Food Storage and Processing, 2nd ed.; Heaps, J.W., Ed.; ACCC International: St. Paul, MN, USA, 2006; pp. 105–146. [Google Scholar]
- White, N.D.G.; Jayas, D.S.; Muir, W.E. Toxicity of carbon dioxide at biologically producible levels to stored-product beetles. Environ. Entomol. 1995, 24, 640–647. [Google Scholar] [CrossRef]
- Lessard, F.F.; LeTorch, J.M. Practical approach to purging grain with low oxygen atmosphere for disinfestation of large wheat bins against the granary weevil, Sitophilus granarius. In Proceedings of the 4nd International Work. Confonference Stored-Product Prot, Tel Aviv, Israel, 21–26 September 1986; pp. 208–217. [Google Scholar]
- Storey, C.L. Exothermic inert atmosphere generator for control of insects in stored wheat. J. Econ. Entomol. 1973, 66, 511–514. [Google Scholar] [CrossRef]
- Mohammed, M.E.; El-Shafie, H.A.; Alhajhoj, M.R. Design and efficacy evaluation of a modern automated controlled atmosphere system for pest management in stored dates. J. Stored Prod. Res. 2020, 89, 101719. [Google Scholar] [CrossRef]
- Catling, D.; Zahnle, K. Evolution of atmospheric oxygen. In Encyclopedia of Atmospheric Sciences; North, G.R., Pyle, J.A., Zhang, F., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 754–761. [Google Scholar]
- Cao, Y.; Gao, S.; Qu, G.; Li, Y.; Li, G.; Carvalho, M.O. Study on the mortality of the stored-grain insects adults in different concentrations of low oxygen. In Proceedings of the 10th International Work. Confonfernece on Stored Product Prot, Estoril, Portugal, 27 June–2 July 2010; Julius Kühn-Archiv: Berlin, Germany, 2010; pp. 476–478. [Google Scholar]
- Bond, E.J.; Buckland, C.T. Development of resistance to carbon dioxide in the granary weevil. J. Econ. Entomol. 1979, 7, 770–771. [Google Scholar] [CrossRef]
- Donahaye, E. Laboratory selection of resistance by the red flour beetle Tribolium castaneum (Herbst) to an atmosphere of low oxygen concentration. Phytoparasitica 1990, 18, 189–202. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhao, Z.M.; Tsai, J.H. Resistance and some enzyme activities in Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae) in relation to carbon dioxide enriched atmospheres. J. Stored Prod. Res. 2020, 36, 297–308. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, K.; Zhu, X.; Bai, Y.; Yang, W.; Li, C. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Buckland, P.C. Granaries, stores and insects. The archeology of insect synanthropy. In La Préparation Alimentaire des Céréales; Fournier, D., Sigaut, F., Eds.; PACT: Rixensart, Belgium, 1990; pp. 69–81. [Google Scholar]
- Lavigne, R. Stored grain insects in underground storage pits in Somalia and their control. Int. J. Trop. Insect Sci. 1991, 12, 571–578. [Google Scholar] [CrossRef]
- Bailey, S.W. Air-tight storage of grain; its effects on insect pests-I. Calandra granaria L. (Coleoptera, Curculionidae). Aust. J. Agric. Res. 1955, 6, 33–51. [Google Scholar] [CrossRef]
- Bailey, S.W. Air-tight storage of grain; its effects on insect pests-IV. Rhyzopertha dominica (F.) and some other Coleoptera that infest stored grain. J. Stored Prod. Res. 1965, 1, 25–33. [Google Scholar]
- Adhikarinayake, T.B.; Palipane, K.B.; Müller, J. Quality change and mass loss of paddy during airtight storage in a ferro-cement bin in Sri Lanka. J. Stored Prod. Res. 2006, 42, 377–390. [Google Scholar] [CrossRef]
- Prasantha, B.D.R.; Kumarasinha, K.M.H.; Emitiyagoda, G.A.M.S. Storage of mungbean in hermetic PVC tank. In Proceedings of the 12th International Work, Conference on Stored Product Prot, Berlin, Germany, 7–11 October 2018; Julius Kühn-Archiv: Berlin, Germany, 2018; pp. 441–447. [Google Scholar]
- Tivana, L.D.; Nguenha, R.J.; Viola, P.; Monjane, I.; Lungu, I.O.; Kafwamfwa, N. Effectiveness of high-density polyethylene container and Super Grain Bag for the storage system of cowpea grain for smallholder farmers. Legum. Sci. 2020, 3e67, 1–12. [Google Scholar]
- Villers, P.; Navarro, S.; Bruin, T. New applications of hermetic storage for grain storage and transport. In Proceedings of the 10th International Work. Conference on Stored Product Protect, Estoril, Portugal, 27 June–2 July 2010; Julius-Kühn-Archiv: Berlin, Germany, 2010; pp. 446–452. [Google Scholar]
- Ochandio, D.C.; Cardoso, L.M.; Bartosik, R.E.; De la Torre, D.A.; Rodríguez, J.C.; Massigoge, J. Storage of quality malting barley in hermetic plastic bags. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Julius-Kühn-Archiv: Berlin, Germany, 2010; pp. 331–337. [Google Scholar]
- Murdock, L.L.; Baoua, I.B. On Purdue improved cowpea storage (PICS) technology: Background, mode of action, future prospects. J. Stored Prod. Res. 2014, 58, 3–11. [Google Scholar] [CrossRef]
- Wilkin, D.R.; Green, A.A. Polythene sacks for the control of insects in bagged grain. J. Stored Prod. Res. 1970, 6, 97–101. [Google Scholar] [CrossRef]
- Guenha, R.; das Virtuedes Salvador, B.; Rickman, J.; Goulao, L.F.; Muocha, I.M.; Carvalho, M.O. Hermetic storage with plastic sealing to reduce insect infestation and secure paddy seed quality: A powerful strategy for rice farmers in Mozambique. J. Stored Prod. Res. 2014, 59, 275–281. [Google Scholar] [CrossRef]
- Sanon, A.; Dabiré-Binso, L.C.; Ba, N.M. Triple-bagging of cowpeas within high density polyethylene bags to control the cowpea beetle Callosobruchus maculatus F. (Coleoptera: Bruchidae). J. Stored Prod. Res. 2011, 47, 210–215. [Google Scholar] [CrossRef]
- Mubayiwa, M.; Mvumi, B.M.; Stathers, T.; Mlambo, S.; Nyabako, T. Field evaluation of hermetic and synthetic pesticide-based technologies in smallholder sorghum grain storage in hot and arid climates. Sci. Rep. 2021, 11, 3692. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Opit, G.P.; Arthur, F.H.; Bingham, G.V.; Payton, M.E.; Gautam, S.G.; Noden, B.H. Effectiveness of the ZeroFly® storage bag fabric against stored-product insects. J. Stored Prod. Res. 2017, 73, 87–97. [Google Scholar] [CrossRef]
- Ngwenyama, P.; Mvumi, B.; Nyanga, L.; Stathers, T.; Siziba, S. Comparative performance of five hermetic bag brands during on-farm smallholder cowpea (Vigna unguiculata L. Walp) storage. J. Stored Prod. Res. 2020, 88, 101658. [Google Scholar] [CrossRef]
- Mutambuki, K.; Affognon, H.; Likhayo, P.; Baributsa, D. Evaluation of Purdue improved crop storage triple layer hermetic storage bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Insects 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Lara, S.; Ortíz-Islas, S.; Villers, P. Portable hermetic storage bag resistant to Prostephanus truncatus, Rhyzopertha dominica, and Callosobruchus maculatus. J. Stored Prod. Res. 2013, 54, 23–25. [Google Scholar] [CrossRef]
- Otitodun, G.O.; Ogundare, M.O.; Ajao, S.K.; Nwaubani, S.I.; Abel, G.I.; Opit, G.P.; Bingham, G.; Omobowale, M.O. Efficacy of phosphine and insect penetration ability in ZeroFly® bags. J. Stored Prod. Res. 2019, 82, 81–90. [Google Scholar] [CrossRef]
- Riudavets, J.; Castane, C.; Alomar, O.; Pons, M.J.; Gabarra, R. Modified atmosphere packaging (MAP) as an alternative measure for controlling ten pests that attack processed food products. J. Stored Prod. Res. 2009, 45, 91–96. [Google Scholar] [CrossRef]
- Kucerova, Z.; Kyhos, K.; Aulicky, R.; Stejskal, V. Low pressure treatment to control food-infesting pests (Tribolium castaneum, Sitophilus granarius) using a vacuum packing machine. Czech J. Food Sci. 2013, 31, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, Z.; Kyhos, K.; Aulicky, R.; Lukas, J.; Stejskal, V. Laboratory experiments of vacuum treatment in combination with an O2 absorber for the suppression of Sitophilus granarius infestations in stored grain samples. Crop Prot. 2014, 61, 79–83. [Google Scholar] [CrossRef]
- Aulicky, R.; Kolar, V.; Plachy, J.; Stejskal, V. Field efficacy of brief exposure of adults of six storage pests to nitrogen-controlled atmospheres. Plant Protect. Sci. 2017, 53, 169–176. [Google Scholar]
- Williams, P.; Minett, W.; Navarro, S.; Amos, T.G. Sealing a farm silo for insect control by nitrogen swamping for fumigation. Aust. J. Exp. Anim. Husb. 1980, 20, 108–114. [Google Scholar] [CrossRef]
- Carvalho, M.O.; Pires, I.; Barbosa, A.; Barros, G.; Riudavets, J.; Garcia, A.C.; Navarro, S. The use of modified atmospheres to control Sitophilus zeamais and Sitophilus oryzae on stored rice in Portugal. J. Stored Prod. Res. 2012, 50, 49–56. [Google Scholar] [CrossRef]
- Morrison, W.R.; Arthur, F.H.; Wilson, L.T.; Yang, Y.; Wang, J.; Athanassiou, C.G. Aeration to manage insects in wheat stored in the Balkan peninsula: Computer simulations using historical weather data. Agronomy 2020, 10, 1927. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Reed, C.; Kenkel, P. Management of stored wheat insect pests in the USA. Integrated Pest Manag. Rev. 1999, 4, 127–143. [Google Scholar] [CrossRef]
- Sthong, R.G.; Sbur, D.E. Protective sprays against internal infestations of grain beetles in wheat. J. Econ. Entomol. 1964, 57, 544–548. [Google Scholar] [CrossRef]
- Cotton, R.T.; Walkden, H.H.; White, G.D.; Wilbur, D.A. Causes of outbreaks of stored-grain insects. In Bulletin 416; Agricultural Experiment Station, Kansas State University of Agriculture and Apllied Science: Manhattan, KS, USA, 1960; p. 35. [Google Scholar]
- Cotton, R.T. Pests of Stored Grain and Stored Products; Burgess Publishing Co.: Minneapolis, MN, USA, 1963. [Google Scholar]
- Arthur, F.H. Grain protectants: Current status and prospect for the future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Rahman, K.; Sohi, G. Mercury as a preventive against stored grain pests. Bull. Entomol. Res. 1946, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Shepard, H.H. Insects Infesting Stored Grain and Seeds. In Bulletin 340; University of Minnesota Agricultural Experiment Station: Saint Paul, MN, USA, 1947; p. 31. [Google Scholar]
- White, N.D.G.; Leesch, J.G. Chemical control. In Integrated Management of Insects in Stored Products; Subramanyam, B., Hagstrum, D.W., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 287–330. [Google Scholar]
- Desmarchelier, J.M.; Banks, H.J.; Williams, P.; Minetta, W. Toxicity of dichlorvos. J. Stored Prod. Res. 1977, 13, 1–12. [Google Scholar] [CrossRef]
- Arthur, F.H. Efficacy of Combinations of methoprene and deltamethrin as long-term commodity protectants. Insects 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddi, K.; Valbon, W.R.; Jumbo, L.O.V.; de Oliveira, L.O.; Guedes, R.N.; Oliveira, E.E. Diversity and convergence of mechanisms involved in pyrethroid resistance in the stored grain weevils. Sitophilus spp. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.S.; Bacca, T.; Silva, A.P.N.; Canal, N.A.; Haddi, K. Control failure and insecticides resistance in populations of Rhyzopertha dominica (Coleoptera: Bostrichidae) from Colombia. J. Stored Prod. Res. 2021, 92, 101802. [Google Scholar] [CrossRef]
- Yue, B.; Wilde, G.E.; Arthur, F. Evaluation of thiamethoxam and imidacloprid as seed treatments to control European corn borer and Indianmeal moth (Lepidoptera: Pyralidae) larvae. J. Econ. Entomol. 2003, 96, 503–509. [Google Scholar] [CrossRef]
- Boukouvala, M.; Kavallieratos, N. Evaluation of two formulations of chlorantraniliprole as maize protectants for the management of Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae). Insects 2021, 12, 194. [Google Scholar] [CrossRef]
- Hertlein, M.B.; Thompson, G.D.; Subramanyam, B.; Athanassiou, C.G. Spinosad: A new natural product for stored grain protection. J. Stored Prod. Res. 2011, 47, 131–146. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Effects of short exposures to spinosad-treated wheat or maize on four stored-grain insects. J. Econ. Entomol. 2010, 103, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilakos, T.N.; Athanassiou, C.G. Effect of short exposures to spinetoram against three stored-product beetle species. J. Econ. Entomol. 2012, 105, 1088–1094. [Google Scholar] [CrossRef]
- Weaver, D.K.; Subramanyam, B. Botanicals. In Alternatives to Pesticides in Stored-Product IPM; Subramanyam, B., Hagstrum, D., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 303–320. [Google Scholar]
- Athanassiou, C.G.; Kontodimas, D.C.; Kavallieratos, N.G.; Veroniki, M.A. Insecticidal effect of NeemAzal against three stored-product beetle species on rye and oats. J. Econ. Entomol. 2005, 98, 1733–1738. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Ntalli, N.; Boukouvala, M.C.; Ntalaka, C.T.; Maggi, F.; Rakotosaona, R.; Cespi, M.; Perinelli, D.R.; et al. Developing a Hazomalania voyronii essential oil nanoemulsion for the eco-friendly management of Tribolium confusum, Tribolium castaneum and Tenebrio molitor larvae and adults on stored wheat. Molecules 2021, 26, 1812. [Google Scholar] [CrossRef]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.D. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 2019, 39, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sujatha, A.; Punnaiah, K.C. Effect of coating stored seed of greengram with vegetable oils on the development of pulse beetle. Indian J. Agric. Sci. 1985, 55, 475–477. [Google Scholar]
- Reed, C.; Pedersen, J.R.; Cuperus, G.W. Efficacy and cost effectiveness of grain protectants applied to farm-stored wheat at harvest and later. J. Econ. Entomol. 1993, 86, 1590–1598. [Google Scholar] [CrossRef]
- Pozidi-Metaxa, E.; Athanassiou, C.G. Comparison of spinosad with three traditional grain protectants against Prostephanus truncatus (Horn) and Ephestia kuehniella (Zeller) at different temperatures. J. Pest Sci. 2013, 86, 203–210. [Google Scholar] [CrossRef]
- Subramanyam, B.; Hagstrum, D.W. Resistance measurement and management. In Integrated Management of Insects in Stored Products; Subramanyam, B., Hagstrum, D.W., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 331–399. [Google Scholar]
- Daglish, G.J.; Eeklema, M.; Harrison, M. Control of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in paddy rice using chlorpyrifos-methyl or fenitrotion in combination with several other protectants. J. Stored Prod. Res. 1996, 32, 247–253. [Google Scholar] [CrossRef]
- Dauguet, S.; Fleurat-Lessard, F.; Loison, J. Cross-contamination of oilseeds by insecticide residues during storage. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Carvalho, M.O., Ed.; Julius Kühn-Archiv: Berlin, Germany, 2010; pp. 827–832. [Google Scholar]
- Williams, C.M. Third-generation pesticides. Sci. Am. 1967, 217, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Pener, M.P.; Dhadialla, T.S. Chapter One—An overview of insect growth disruptors; applied aspects. In Advances in Insect Physiology; Dhadialla, T.S., Ed.; Insect Growth Disruptors; Academic Press: Cambridge, MA, USA, 2012; Volume 43, pp. 1–162. [Google Scholar]
- Wijayaratne, L.K.W.; Fields, P.G.; Arthur, F.H. Residual efficacy of methoprene for control of Tribolium castaneum (Coleoptera: Tenebrionidae) larvae at different temperatures on varnished wood, concrete, and wheat. J. Econ. Entomol. 2012, 105, 718–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daglish, G.J.; Pulvirenti, C. Reduced fecundity of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) following exposure of adults to methoprene. J. Stored Prod. Res. 1997, 34, 201–206. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Vayias, B.J.; Tomanovic, Z. Efficacy of insect growth regulators as grain protectants against two stored-product pestsin wheat and maize. J. Food Prot. 2012, 75, 942–950. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Daglish, G.J.; Nayak, M.K. Uneven application can influence the efficacy of S-methoprene against Rhyzopertha dominica on wheat. J. Stored Prod. Res. 2010, 46, 250–253. [Google Scholar] [CrossRef]
- Edde, P.A. Review of the biology and control of Rhyzopertha dominica (F.) the lesser grain borer. J. Stored Prod. Res. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Arthur, F.H. Efficacy of methoprene for multi-year protection of stored wheat, brown rice, rough rice and corn. J. Stored Prod. Res. 2016, 68, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Daglish, G.J. Efficacy of six grain protectants applied alone or in combination against three species of Coleoptera. J. Stored Prod. Res. 1998, 34, 263–268. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Arthur, F.H.; Throne, J.E. Efficacy of spinosad and methoprene, applied alone or in combination, against six stored product insect species. J. Pest Sci. 2011, 84, 61–67. [Google Scholar] [CrossRef]
- Lui, S.; Arthur, F.H.; Van Gundy, D.; Phillips, T.W. Combination of methoprene and controlled aeration to manage insects in stored wheat. Insects 2016, 7, 25. [Google Scholar]
- Reed, C. Managing Stored Grain to Preserve Quality and Value; American Association of Cereal Chemists, ACCC International: St. Paul, MN, USA, 2006. [Google Scholar]
- Minett, W.; Williams, P. Influence of malathion distribution on the protection of wheat grain against insect infestation. J. Stored Prod. Res. 1971, 7, 233–242. [Google Scholar] [CrossRef]
- Subramanyam, B.; Boina, D.R.; Sehgal, B.; Lazzari, F. Efficacy of partial treatment of wheat with spinosad against Rhyzopertha dominica (F.) adults. J. Stored Prod. Res. 2014, 59, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H. Efficacy of deltamethrin against stored-product beetles at short exposure intervals or on a partially treated rice mass. J. Econ. Entomol. 2015, 108, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Scully, E.D.; Gerken, A.R.; Fifield, A.; Nguyen, V.; Van Pelt, N.; Arthur, F.H. Impacts of Storicide II on internal feeders of Brown rice. J. Stored Prod. Res. 2021, 90, 101758. [Google Scholar] [CrossRef]
- Singh, S.K.; Fan, L.T. A biopolymer-based pesticide delivery system for insect suppression in stored grains. Pestic. Sci. 1989, 25, 273–288. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Dutton, A.C.; Athanassiou, C.G. Insecticidal efficacy of two pirimiphos-methyl formulations for the control of three stored-product beetle species: Effect of commodity. Crop Protect. 2016, 80, 94–100. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Nika, E.P.; Boukouvala, M.C. Efficacy of alpha-cypermethrin, chlorfenapyr and pirimiphos-methyl applied on polypropylene bags for the control of Prostephanus truncatus (Horn), Rhyzopertha dominica (F.) and Sitophilus oryzae (L.). J. Stored Prod. Res. 2017, 73, 54–61. [Google Scholar] [CrossRef]
- Szilagyi, J. NovIGRain project aims to cut losses of stored grain. Int. Pest Control 2021, 63, 102–103. [Google Scholar]
- Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Efficacy of spinosad in layer-treated wheat against five stored-product insect species. J. Stored Prod. Res. 2009, 45, 236–240. [Google Scholar] [CrossRef]
- Golob, P.; Hanks, C. Protection of farm stored maize against infestation by Prostephanus truncatus (Horn) and Sitophilus species in Tanzania. J. Stored Prod. Res. 1990, 26, 187–198. [Google Scholar] [CrossRef]
- Hodges, R.J.; Meik, J. Infestation of maize cobs by Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) Aspects of biology and control. J. Stored Prod. Res. 1984, 20, 205–213. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Khan, Y.S.A. Insects infestation and preventive measures in dry fish storage of Chittagong, Bangladesh. Int. J. Biol. Sci. 2001, 1, 963–965. [Google Scholar]
- Rajendran, S.; Parveen, K.M.H. Insect infestation in stored animal products. J. Stored Prod. Res. 2005, 41, 1–30. [Google Scholar] [CrossRef]
- MacQuillan, M.J.; Shipp, E. Evaluation of chlorpyrifos and chlorpyriphos-methyl for control of Dermestes maculatus Deg. (Coleoptera: Dermestidae) on sheepskins. J. Stored Prod. Res. 1976, 12, 93–96. [Google Scholar] [CrossRef]
- Golob, P.; Cox, J.R.; Kilminster, K. Evaluation of insecticide dips as protectants of stored dried fish from dermestid beetle infestation. J. Stored Prod. Res. 1987, 23, 47–56. [Google Scholar] [CrossRef]
- Don-Pedro, K.N. Toxicity of some citrus peels to Dermestes maculatus Deg. and Callosobruchus maculatus (F). J. Stored Prod. Res. 1985, 21, 31–34. [Google Scholar] [CrossRef]
- Onu, I.; Baba, G.O. Evaluation of Neem products for the control of Dermestid beetle on dried fish. Niger. J. Entomol. Soc. 2003, 20, 105–115. [Google Scholar]
- Nowsad, A.K.M.; Mondal, R.; Islam, M.R. Effectiveness of neem, garlic and red chili against adult dermestid beetle in sun dried fish. Progress. Agric. 2013, 20, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Golob, P.; Gueye-Ndiaye, A.; Johnson, S. Prevention of beetle infestation of dried fish. In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Wallingford, UK, 1994; pp. 777–781. [Google Scholar]
- Pretoru, E.G. Studies on Control of Dermestes maculatus Degeer on Dried Fish Treated by ULV Application. Ph.D. Thesis, Imperial College London, Silwood Park, UK, 1988. [Google Scholar]
- Khan, Y.S.A.; Khan, M.A.A. Study on the use of DDT as preservative practiced in stored dried fishes of Bangladesh. Poll. Res. 1998, 17, 363–365. [Google Scholar]
- Islam, M.N.; Kabir, M.A. Application of organic preservatives for sustainable storage of dried fishes. Int. J. Fish. Aquat. 2019, 7, 40–43. [Google Scholar]
- Adebote, D.A.; Abolude, D.S.; Oniye, S.J.; Olododo, S.S.; Hassan, M.M. Larvicidal and repellent actions of Detarium microcarpum seed oil against the larvae of Dermestes lardarius (Coleoptera: Dermestidae) in dried Clarias gariepinus fish. J. Entomol. 2006, 3, 248–253. [Google Scholar]
- Don-Pedro, K.N. Insecticidal activity of some vegetable oils against Dermestes maculatus Degeer (Coleoptera: Dermestidae) on dried fish. J. Stored Prod. Res. 1989, 25, 81–86. [Google Scholar] [CrossRef]
- Idris, G.L.; Omojowo, F.S. Comparative study of groundnut oil and sodium chloride as protectants against insect infestation of smoked dried fish in Kainji lake areas. J. Fish. Aquat. Sci. 2013, 8, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 1017–1024. [Google Scholar] [CrossRef]
- Rust, M.K.; Choe, D.H.; Wilson-Rankin, E.; Campbell, K.; Kabashima, J.; Dimson, M. Controlling yellow jackets with fipronil-based protein baits in urban recreational areas. Int. J. Pest Manag. 2017, 63, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Wongthangsiri, D.; Pereira, R.M.; Bangs, M.J.; Koehler, P.G.; Chareonviriyaphap, T. Potential of attractive toxic sugar baits for controlling Musca domestica L., Drosophila melanogaster Meigen, and Megaselia scalaris Loew adult flies. Agric. Nat. Res. 2018, 52, 393–398. [Google Scholar] [CrossRef]
- Gore, J.C.; Schal, C. Laboratory evaluation of boric acid-sugar solutions as baits for management of German cockroach infestations. J. Econ. Entomol. 2004, 97, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.H.; Campbell, K.; Hoddle, M.S.; Kabashima, J.; Dimson, M.; Rust, M.K. Evaluation of a hydrogel matrix for baiting western yellowjacket (Vespidae: Hymenoptera). J. Econ. Entomol. 2018, 111, 1799–1805. [Google Scholar] [CrossRef]
- Kikuta, S. Deployment of an attractive toxic sugar bait system (ATSB) with insecticide, for adult Tribolium castaneum (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2019, 83, 97–102. [Google Scholar] [CrossRef]
- Lee, S.H.; Choe, D.H.; Lee, C.Y. The impact of artificial sweeteners on insects. J. Econ. Entomol. 2021, 114, 1–13. [Google Scholar] [CrossRef]
- Baudier, K.M.; Kaschock-Marenda, S.D.; Patel, N.; Diangelus, K.L.; O’Donnell, S.; Marenda, D.R. Erythritol, a non-nutritive sugar alcohol sweetener and the main component of Truvia®, is a palatable ingested insecticide. PLoS ONE 2014, 9, e98949. [Google Scholar]
- Barrett, M.; Caponera, V.; McNair, C.; O’Donnell, S.; Marenda, D.R. Potential for use of erythritol as a socially transferrable ingested insecticide for ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2020, 113, 1382. [Google Scholar] [CrossRef]
- Díaz-Fleischer, F.; Arredondo, J.; Lasa, R.; Bonilla, C.; Debernardi, D.; Pérez-Staples, D.; Williams, T. Sickly sweet: Insecticidal polyols induce lethal regurgitation in dipteran pests. Insects 2019, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Perkin, L.C.; Adrianos, S.L.; Oppert, B. Gene disruption technologies have the potential to transform stored product insect pest control. Insects 2016, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Huang, J.H.; Liu, Y.; Lin, Y.H.; Belles, X.; Lee, H.J. Practical use of RNA interference: Oral delivery of double-stranded RNA in liposome carriers for cockroaches. J. Vis. Exp. 2018, 135, e57385. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Miao, S.; Yang, B.; Wang, Z.; Liu, Q.; Wang, R.; Du, X.; Ren, Y.; Lu, Y. Initial characterization of the vitellogenin receptor from a Psocoptera insect: Function analysis and RNA interference in Liposcelis entomophila (Enderlein). J. Stored Prod. Res. 2021, 92, 101803. [Google Scholar] [CrossRef]
- Stejskal, V.; Aulicky, R.; Kucerova, Z. Pest control strategies and damage potential of seed-infesting pests in the Czech stores—a review. Plant Protect. Sci. 2014, 50, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Athanassiou, C.G.; Riudavets, J.; Kavallieratos, N.G. Preventing stored-product insect infestations in packaged-food products. Stewart Postharvest Rev. 2011, 3, 1–5. [Google Scholar]
- Yadav, T.D. Evaluation of deltamethrin as fabric treatment in storage of wheat seed at farm level. Indian J. Entomol. 1997, 59, 103–109. [Google Scholar]
- Cotton, R.T.; Balzer, A.I.; Young, H.D. Possible utility of DDT for insect-proofing paper bags. J. Econ. Entomol. 1944, 37, 140. [Google Scholar] [CrossRef]
- Hayhurst, H. The action on certain insects of fabrics impregnated with DDT. J. Soc. Chem. Ind. 1945, 64, 296. [Google Scholar] [CrossRef]
- Parkin, E.A. D.D.T. impregnation of sacks for the protection of stored cereals against insect infestation. Ann. Appl. Biol. 1948, 35, 233–242. [Google Scholar] [CrossRef]
- Atkins, W.G. Storage of flour in jute bags treated with insecticides. J. Sci. Food Agric. 1953, 4, 155–160. [Google Scholar] [CrossRef]
- Prevett, P.F. Treatment of rice stored in jute bags against insect pests. Trop. Stored Prod. Info. 1960, 1, 4–9. [Google Scholar]
- Pali, N.S. Studies on jute bag impregnation for the control of graminivorous insects. J. Entomol. 1961, 22, 211–213. [Google Scholar]
- Webley, D.J.; Kilminster, K.M. The persistence of insecticide spray deposits on woven polypropylene and jute sacking. Pestic. Sci. 1980, 11, 667–673. [Google Scholar] [CrossRef]
- Rai, R.S.; Lal, P.; Srivastava, P.K. Impregnation of jute bags with insecticide for protecting stored food grains. III. Comparative efficacy of impregnation method vis-a-vis existing method of prophylactic chemical treatment against cross infestation of different stored grain insect pests. Pesticides 1987, 21, 39–42. [Google Scholar]
- Yadav, T.D.; Singh, S. Persistence toxicity and efficacy of four insecticides as jute fabric treatment to protect cereal and legume seeds. Indian J. Entomol. 1994, 56, 146–155. [Google Scholar]
- Pathak, K.A.; Jha, A.N.; Singh, J.P. Effect of fabric treatment of jute and polypropylene bags with some insecticides on maize and paddy stored at Delhi and Meghalaya. Shashpa 2002, 9, 61–70. [Google Scholar]
- Morallo-Rejesus, B.; Varca, L.M.; Nerona, E.H. Insecticide impregnation of sacks and use of plastic lining for the protection of stored corn against insect damage. Philipp. Agric. 1975, 59, 196–204. [Google Scholar]
- Webley, D.J.; Kilminster, K.M. The persistence and activity of insecticide spray deposits on woven polypropylene fabric. Pestic. Sci. 1981, 12, 74–78. [Google Scholar] [CrossRef]
- Barakat, A.A.; Khan, P.; Karim, A.A. The persistence and activity of permethrin and chlorpyrifos-methyl sprays on jute and woven polypropylene bags. J. Stored Prod. Res. 1987, 23, 85–90. [Google Scholar] [CrossRef]
- Scheff, D.S.; Subramanyam, B.; Arthur, F.H. Susceptibility of Tribolium castaneum and Trogoderma variabile larvae and adults exposed to methoprene-treated woven packaging material. J. Stored Prod. Res. 2017, 73, 142–150. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kavallieratos, N.G.; Boukouvala, M.C.; Malesios, C. (Quasi)-binomial vs. gaussian models to evaluate thiamethoxam, pirimiphos-methyl, alpha-cypermethrin and deltamethrin on different types of storage bag materials against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) and Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae). Insects 2021, 12, 182. [Google Scholar]
- Himel, C.M. The optimum size for insecticide spray droplets. J. Econ. Entomol. 1969, 62, 919–925. [Google Scholar] [CrossRef]
- WHO. Equipment for Vector Control; WHO: Geneva, Switzerland, 1964. [Google Scholar]
- Sugiura, M.; Horibe, Y.; Kawada, H.; Takagi, M. Effect of different droplet size on the knockdown efficacy of directly sprayed insecticides. Pest. Manag. Sci. 2011, 67, 1115–1123. [Google Scholar] [CrossRef]
- Hewlett, P.S. A direct spray technique for the biological evaluation of pyrethrum-in-oil insecticides for use against stored product insects in warehouses. Ann. Appl. Biol. 1947, 34, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Stadler, T.; Buteler, M. Modes of entry of petroleum distilled spray-oils into insects: A review. Bull. Insectol. 2009, 62, 169–177. [Google Scholar]
- Sugiura, M.; Horibe, Y.; Kawada, H.; Takagi, M. Insect spiracle as the main penetration route of pyrethroids. Pestic. Biochem. Physiol. 2008, 91, 135–140. [Google Scholar] [CrossRef] [Green Version]
- David, W.A.L. The quantity and distribution of spray collected by insects flying through insecticidal mists. Ann. Appl. Biol. 1946, 33, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Carron, A.; Darriet, F.; Etienne, M.; Agnew, P.; Tolosa, M.; Yp-Tcha, M.M.; Lagneau, C.; Yébakima, A.; Corbel, V. Reduced efficacy of pyrethroid space sprays for dengue control in an area of Martinique with pyrethroid resistance. Am. J. Trop. Med. Hyg. 2009, 80, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonds, J.A.S. Ultra-low-volume space sprays in mosquito control: A critical review. Med. Vet. Entomol. 2012, 26, 121–130. [Google Scholar] [CrossRef]
- David, W.A.L. Factors influencing the interaction of insecticidal mists and flying insects. Part II. The production and behaviour of kerosene base insecticidal spray mists and their relation to flying insects. Bull. Entomol. Res. 1946, 37, 1–28. [Google Scholar] [CrossRef]
- Webley, D. Manual of Pest Control for Food Security Reserve Grain Stocks. In FAO Plant Production and Protection Paper No. 63; Food & Agriculture Org: Rome, Italy, 1988; p. 160. [Google Scholar]
- Matthews, G. Space spray fundamentals. Int. Pest Control 2013, 55, 78–80. [Google Scholar]
- Childs, D.P.; Phillips, G.L.; Press, A.R. Control of the cigarette beetle in tobacco warehouses with automatic dichlorvos aerosol treatments. J. Econ. Entomol. 1966, 59, 261–264. [Google Scholar] [CrossRef]
- Suss, L. A new method for the control of insects in warehouses and food industries. In Proceedings of the 7th International Work. Conference on Stored-Product Protection, Beijing, China, 14–19 October 1998; Jin, Z., Liang, Q., Liang, Y., Tan, X., Guan, L., Eds.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1998; pp. 950–954. [Google Scholar]
- Lofgren, C.S. Ultralow volume applications of concentrated insecticides in medical and veterinary entomology. Ann. Rev. Entomol. 1970, 15, 321–342. [Google Scholar] [CrossRef]
- LaMer, V.K.; Hochberg, S.; Hodges, K.; Wilson, I.; Fales, J.A.; Latta, R. The influence of the particle size of homogeneous insecticidal aerosols on the mortality of mosquitoes in confined atmospheres. J. Colloid. Sci. 1947, 2, 539–549. [Google Scholar] [CrossRef]
- Rickett, F.E.; Chadwick, P.R. Measurements of temperature and degradation of pyrethroids in two thermal fogging machines, the Swingfog and TIFA. Pestic. Sci. 1972, 3, 263–269. [Google Scholar] [CrossRef]
- Hoffmann, W.C.; Walker, T.W.; Fritz, B.K.; Gwinn, T.; Smith, V.L.; Szumlas, D.; Quinn, B.; Lan, Y.; Huang, Y.; Sykes, D. Spray characterization of thermal fogging equipment typically used in vector control. J. Am. Mosq. Control Assoc. 2008, 24, 550–559. [Google Scholar] [CrossRef]
- Asuncion, F.X.B.; Brabec, D.L.; Casada, M.E.; Maghirang, R.G.; Arthur, F.H.; Campbell, J.F.; Zhu, K.Y.; Daniel, E.; Martin, D.E. Spray Characterization of aerosol delivery systems for use in stored product facilities. Trans. ASABE 2020, 63, 1925–1937. [Google Scholar] [CrossRef]
- Potter, C. An account of the constitution and use of an atomized white oil-pyrethrum fluid to control Plodia interpunctella and Ephestia elutella in warehouses. Ann. Appl. Biol. 1935, 22, 769–805. [Google Scholar] [CrossRef]
- Potter, C.; Graham-Bryce, I.J. An Appreciation. Ann. Appl. Biol. 1990, 117, 233–235. [Google Scholar] [CrossRef]
- Cornwell, P.B. The Cockroach. Vol. Ii. Insecticides and Cockroach Control; St. Martin’s Press: New York, NY, USA, 1976. [Google Scholar]
- Cogburn, R.R.; Simonaitis, R.A. Dichlorvos for control of stored-product insects in port warehouses: Low-volume aerosols and commodity residues. J. Econ. Entomol. 1975, 68, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Bell, C. Pest control: Insects and mites. In Hygiene in Food Processing; Lelieveld, H.L.M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2003; pp. 335–379. [Google Scholar]
- Scheff, D.S.; Brabec, D.; Campbell, J.F.; Arthur, H.F. Case study: A practical application of an aerosol treatment in a commercial mill. Insects 2019, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Arthur, F.H.; Scheff, D.S.; Brabec, D.; Bindel, J. Aerosol concentration, deposition, particle size, and exposure interval as mortality factors Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2019, 83, 191–199. [Google Scholar] [CrossRef]
- Arthur, F.H.; Campbell, J.F.; Ducatte, G.R. Susceptibility of Tribolium confusum (Coleoptera: Tenebrionidae) to pyrethrin aerosol: Effects of aerosol particle size, concentration, and exposure conditions. J. Econ. Entomol. 2014, 107, 2239–2251. [Google Scholar] [CrossRef]
- Arthur, F.H.; Campbell, J.F.; Brabec, D.L.; Ducatte, G.R.; Donaldson, J.E. Aerosol insecticide distribution inside a flour mill: Assessment using droplet measurements and bioassays. J. Stored Prod. Res. 2018, 77, 26–33. [Google Scholar] [CrossRef]
- Arthur, F.H.; Subramanyam, B.H. Chemical control in stored products. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University Agricultural Experiment Station and Cooperative Extension Service: Manhattan, NY, USA, 2012; pp. 95–100. [Google Scholar]
- Arthur, F.H. Aerosols and contact insecticides as alternatives to methyl bromide in flour mills food production facilities and food warehouses. J. Pest Sci. 2012, 85, 323–329. [Google Scholar] [CrossRef]
- David, W.A.L. Insecticidal sprays and flying insects. Nature 1945, 155, 204. [Google Scholar] [CrossRef]
- David, W.A.L.; Bracey, P. Factors influencing the interaction of insecticidal mists on flying insects: Part III. Biological Factors. Bull. Entomol. Res. 1946, 37, 177–190. [Google Scholar] [CrossRef]
- Opit, G.P.; Arthur, F.H.; Throne, J.E.; Payton, M.E. Susceptibility of stored-product psocids to aerosol insecticides. J. Insect Sci. 2012, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Arthur, F.H.; Campbell, J.F.; Donaldson, J.E. Particle size matters: Efficacy of aerosols for the control of stored product psocids. J. Stored Prod. Res. 2019, 83, 148–152. [Google Scholar] [CrossRef]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Efficacy of methoprene applied at different temperatures and rates to different surface substrates to control eggs and fifth instars of Plodia interpunctella Hübner. J. Econ. Entomol. 2009, 102, 1992–2002. [Google Scholar] [CrossRef]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Efficacy of an esfenvalerate plus methoprene aerosol for the control of eggs and fifth instars of Plodia interpunctela (Lepidoptera: Pyralidae). Insect Sci. 2010, 17, 21–28. [Google Scholar] [CrossRef]
- Fontenot, E.A.; Arthur, F.H.; Nechols, J.R.; Throne, J.E. Using a population growth model to simulate response of Plodia interpunctella Hübner populations to timing and frequency of insecticide treatments. J. Pest Sci. 2012, 85, 469–476. [Google Scholar] [CrossRef]
- Reierson, D.A. Field tests to control German cockroaches with ULV aerosol generators. Pest Control 1973, 41, 26–32. [Google Scholar]
- Moore, R.C. Field tests of pyrethrins and resmethrin applied with ULV generators or total release aerosols to control the German cockroach. J. Econ. Entomol. 1977, 70, 86–88. [Google Scholar] [CrossRef]
- Chadwick, P.R.; Shaw, R.D. Cockroach control in sewers in Singapore using bioresmethrin and piperonyl butoxide as a thermal fog. Pestic. Sci. 1974, 5, 691–701. [Google Scholar] [CrossRef]
- DeVries, Z.C.; Santangelo, R.G.; Crissman, J.; Mick, R.; Schal, C. Exposure risks and ineffectiveness of total release foggers (TRFs) used for cockroach control in residential settings. BMC Public Health 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaakeh, W.; Bennett, G.W. Evaluation of trapping and vacuuming compared with low-impact insecticide tactics for managing German cockroaches in residences. J. Econ. Entomol. 1997, 90, 976–982. [Google Scholar] [CrossRef]
- Wang, C.; Bennett, G.W. Comparative study of integrated pest management and baiting for German cockroach management in public housing. J. Econ. Entomol. 2006, 99, 879–885. [Google Scholar] [CrossRef]
- Small, G.J. A comparison between the impact of sulfuryl fluoride and methyl bromide fumigations on stored-product insect populations in UK flour mills. J. Stored Prod. Res. 2007, 43, 410–416. [Google Scholar] [CrossRef]
- Holland, J.M.; Jepson, P.C. Droplet dynamics and initial field tests for microencapsulated pesticide formulations applied at ultra low volume using rotary atomizers for control of locusts and grasshoppers. Pestic. Sci. 1996, 48, 125–134. [Google Scholar] [CrossRef]
- Einam, J. Understanding Fogging with Water-Based Formulations. Professional Pest Manager, 1 November 2019. BD Publications Pty Ltd ATF BDP Trust ABN 68 191 770 967. Available online: https://professionalpestmanager.com/understanding-fogging-with-water-based-formulations/ (accessed on 17 December 2020).
- Robinson, W.H. The Service Technician’s Application and Equipment Manual: A Practical Guide for Pest Control Professionals; PCT–Pest Control Technology: Valley View, OH, USA, 2011; p. 128. [Google Scholar]
- Aulicky, R.; Stejskal, V. Vertical and horizontal distribution of pesticide microcapsules applied on the porous surface. Res. Agric. Eng. 2002, 48, 153–157. [Google Scholar]
- Potter, C. The use of protective films of insecticide in the control of indoor insects, with special reference to Plodia interpunctella Hb. and Ephestia elutella Hb. Ann. Appl. Biol. 1938, 25, 836–854. [Google Scholar] [CrossRef]
- Pradhan, S. Studies on the toxicity of insecticide films: I. Preliminary investigations on concentration-time-mortality relation. Bull. Entomol. Res. 1949, 40, 1–25. [Google Scholar] [CrossRef]
- Shafer, G.D. How Contact Insecticides Kill. III; Technical Bulletin Michigan Agriculture Experimental Station No 21: East Lansing, MI, USA, 1915; p. 67. [Google Scholar]
- Tattersfield, F.; Potter, C. Biological methods for determining the insecticidal values of pyrethrum preparations (particularly extracts in heavy oil). Ann. Appl. Biol. 1943, 30, 259–279. [Google Scholar] [CrossRef]
- Parkin, E.A.; Green, A.A. A film technique for the biological evaluation of pyrethrum-in-oil insecticides for use agaiarnst stored product insects in warehouses. Ann. Appl. Biol. 1943, 30, 279–292. [Google Scholar] [CrossRef]
- Busvine, J.R.; Barnes, S. Observations on mortality of insects exposed to dry insecticidal films. Bull. Entomol. Res. 1947, 38, 81–90. [Google Scholar] [CrossRef]
- Lindquist, A.W.; Wilson, H.G.; Schroeder, H.O.; Madden, A.H. Effect of temperatures on knockdown and kill of houseflies exposed to DDT. J. Econ. Entomol. 1945, 38, 261–264. [Google Scholar] [CrossRef]
- Swenk, M.H. Insect Pests of Stored Grains and Their Control; Agricultural Experiment Station, The University of Nebraska: Lincoln, NE, USA, 1922. [Google Scholar]
- Zacher, F. Die Vorrats-, Speicher- und Materialschadlinge und ihre Bekampfung; Verlagsbuchhandlung Paul Parey: Berlin, Germany, 1927; p. 366. [Google Scholar]
- Robinson, R.H. Sprays. Their preparation and use. Oregon Ext. Bull. 1941, 93, 8–16. [Google Scholar]
- McDaniel, E.I. Pest control in dairies. J. Milk Food Technol. 1945, 8, 338–341. [Google Scholar] [CrossRef]
- Rao, N.V.S.; Pollard, A.G. Photo-decomposition of rotenone in spray deposits. III.—Kinetics of the photo-decomposition. J. Sci. Food Agric. 1951, 2, 462–472. [Google Scholar] [CrossRef]
- Scott, G.H.; Lettig, K.S. Flies of Public Health Importance and Their Control; U.S. Government Printing Office: Washington, DC, USA, 1962.
- Murphy, D.F. Combination Insecticide Composition. United States Patent Office 194033061, 25 July 1935. [Google Scholar]
- Brooks, G.T. Chlorinated Insecticides; CRC Press: Cleveland, OH, USA, 1974; Volume 1, p. 102. [Google Scholar]
- Bennett, G.W.; Reid, B.L. Insect growth regulators. In Understanding and Controlling the German Cockroach, 1st ed.; Rust, M.K., Owens, J., Reierson, D.A., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 267–286. [Google Scholar]
- Kavallieratos, N.G.; Boukouvala, M.C. Efficacy of d-tetramethrin and acetamiprid for control of Trogoderma granarium Everts (Coleoptera: Dermestidae) adults and larvae on concrete. J. Stored Prod. Res. 2019, 80, 79–84. [Google Scholar] [CrossRef]
- Kavallieratos, N.; Athanassiou, C.; Barda, M.; Boukouvala, M. Efficacy of five insecticides for the control of Trogoderma granarium Everts (Coleoptera: Dermestidae) larvae on concrete. J. Stored Prod. Res. 2016, 66, 18–24. [Google Scholar] [CrossRef]
- Arthur, F.H. Efficacy of chlorfenapyr against Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) adults exposed on concrete, vinyl tile, and plywood surfaces. J. Stored Prod. Res. 2008, 44, 145–151. [Google Scholar] [CrossRef]
- Robinson, W.H. Technologies-how it works: Less droplets, same effectiveness on cockroaches. Pest Control 2005, 73, 114–116. [Google Scholar]
- Hodges, R.J.; Dales, M.J. Report on an investigation of insecticide persistence on grain store surfaces in Ghana. NRI Report 1991, 2630, 1–26. [Google Scholar]
- Harein, P.K. Chemical control of insect pests in bulk-stored grains. In Ecology and Management of Food Industry Pests. FDA Technical Bulletin 4; Gorham, J.R., Ed.; Association of Analytical Chemists: Arlington, VG, USA, 1991; pp. 415–418. [Google Scholar]
- Wickham, J.C. Conventional insecticides. In Understanding and Controlling the German Cockroach; Rust, M.K., Owens, J.M., Reierson, D.A., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 109–147. [Google Scholar]
- Ross, M.H. Comparisons between the response of German cockroach field-collected strains (Dictyoptera: Blattellidae) to vapors and contact with a cyfluthrin formulation. J. Entomol. Sci. 1993, 28, 168–174. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K. Microencapsulation of pesticides and their improved handling safety. J. Microencapsul. 2001, 18, 137–147. [Google Scholar] [CrossRef]
- Stejskal, V.; Aulicky, R.; Pekar, S. Brief exposure of Blattella germanica (Blattodea) to insecticides formulated in various microcapsule sizes and applied on porous and non-porous surfaces. Pest Manag. Sci. 2009, 65, 93–98. [Google Scholar] [CrossRef]
- Zou, A.; Yang, Y.; Cheng, J.; Vasil, M.; Garamus, V.M.; Li, N. Construction and characterization of a novel sustained-release delivery system for hydrophobic pesticides using biodegradable polydopamine-based microcapsules. J. Agric. Food Chem. 2018, 66, 6262–6268. [Google Scholar] [CrossRef]
- Reid, J.A. A small trial of insecticidal resins for control of cockroaches. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 227–231. [Google Scholar] [CrossRef]
- Price, M.D. Insecticidal resins. A new concept of insect control. Pest Control 1960, 28, 47–58. [Google Scholar]
- Khoobdel, M.; Ahsaei, S.M.; Farzaneh, M. Insecticidal activity of polycaprolactone nanocapsules loaded with Rosmarinus officinalis essential oil in Tribolium castaneum (Herbst). Entomol. Res. 2017, 47, 175–184. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Abd El-Aziz, S.E.S. Impact of certain nano oils against Ephestia kuehniella and Ephestia cutella (Lepidoptera-Pyralidae) under laboratory and store conditions. Bull. Natl. Res. Cent. 2019, 43, 1–7. [Google Scholar] [CrossRef]
- Shirley, I.M.; Scher, H.B.; Perrin, R.M.; Wege, P.J.; Rodson, M.; Chen, J.L.; Rehmke, A.W. Delivery of biological performance via micro-encapsulation formulation chemistry. Pest Manag. Sci. 2001, 57, 129–132. [Google Scholar] [CrossRef]
- Peterson, C.; Coats, J. Insect repellents—Past, present and future. Pestic. Outlook 2001, 12, 154–158. [Google Scholar] [CrossRef]
- Gaire, S.; O’Connell, M.; Holguin, F.O.; Amatya, A.; Bundy, S.; Romero, A. Insecticidal properties of essential oils and some of their constituents on the Turkestan cockroach (Blattodea: Blattidae). J. Econ. Entomol. 2017, 110, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Nalyanya, G.; Moore, C.B.; Schal, C. Integration of repellents, attractants, and insecticides in a “push-pull” strategy for managing German cockroach (Dictyoptera: Blattellidae) populations. J. Med. Entomol. 2000, 37, 427–434. [Google Scholar]
- Zhu, J. Push and pull strategy in control of filth flies in urban settings. In Proceedings of the 2012 National Conference on Urban Entomology, Atlanta, GA, USA, 20–23 July 2012; Suiter, D., Ed.; USDA ARS: Washington, DC, USA, 2012; p. 168. [Google Scholar]
- Brenner, R.J.; Milne, D.E.; Kinscherf, K.M.; Connors, T.F. Measuring spatial displacement of Blattella germanica (Blattaria: Blattellidae) populations pressured by repellent treated harborages. Environ. Entomol. 1998, 27, 10–21. [Google Scholar] [CrossRef]
- Peterson, C.J.; Fristad, A.; Tsao, R.; Coats, J.R. Examination of osage orange fruits and two isoflavones, osajin and pomiferin, for repellency to the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Environ. Entomol. 2000, 29, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Sharififard, M.; Safdari, F.; Siahpoush, A.; Kassiri, H. Evaluation of some plant essential oils against the brown-banded cockroach, Supella longipalpa (Blattaria: Ectobiidae): A mechanical vector of human pathogens. J. Arthropod Borne. Dis. 2016, 10, 528–537. [Google Scholar]
- Oladipupo, S.O.; Hu, X.P.; Appel, A.G. Topical toxicity profiles of some aliphatic and aromatic essential oil components against insecticide-susceptible and resistant strains of German cockroach (Blattodea: Ectobiidae). J. Econ. Entomol. 2020, 113, 896–904. [Google Scholar] [CrossRef]
- Karr, L.L.; Coats, J.R. Repellency of dried bay leaves (Laurus nobilis), Wrigley’s© spearmint gum, raw Osage orange fruit (Maclura pomifera), and extracts of Osage orange fruit to the German cockroach. Insectic. Acaric. Tests 1991, 17, 393. [Google Scholar]
- Appel, A.G.; Mack, T.P. Repellency of milled aromatic eastern red cedar to domiciliary cockroaches (Dictypotera: Blattellidae and Blattidae). J. Econ. Entomol. 1989, 82, 152–155. [Google Scholar] [CrossRef]
- Rust, M.K. Cockroaches. In Public Health Significance of Urban Pests; Bonnefoy, X., Kampen, H., Sweeney, K., Eds.; World Health Organization: Geneva, Switzerland, 2008; pp. 53–84. Available online: http://www.euro.who.int/_data/assets/pdf_Þle/0011/98426/E91435.pdf (accessed on 24 June 2021).
- Oi, D.H.; Vail, K.M.; Williams, D.F. Bait distribution among multiple colonies of Pharaoh ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2000, 93, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H. Methodology for evaluating the insect growth regulator (IGR) methoprene on packaging film. Insects 2016, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Arthur, F.H.; Starkus, L.A.; McKay, T. Degradation and residual efficacy of cyfluthrin as a surface treatment for control of Tribolium castaneum Herbst: Effects of temperature and environment. J. Stored Prod. Res. 2019, 84, 101514. [Google Scholar] [CrossRef]
- Arthur, F.H.; Athanassiou, C.G.; Morrison III, W.R. Mobility of stored product beetles after exposure to a combination insecticide containing deltamethrin, methoprene, and a piperonyl butoxide synergist depends on species, concentration, and exposure time. Insects 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karanika, C.; Rumbos, C.; Agrafioti, P.; Athanassiou, C. Insecticidal efficacy of a binary combination of cyphenothrin and prallethrin, applied as surface treatment against four major stored-product insects. J. Stored Prod. Res. 2019, 80, 41–49. [Google Scholar] [CrossRef]
- Arthur, F.H.; Peckman, P. Insect management with residual insecticides. In Insect Management for Food Storage and Processing; Heaps, J.W., Ed.; ACCC International: St. Paul, MN, USA, 2006; pp. 167–173. [Google Scholar]
- Guedes, R.N.C.; Campbell, J.F.; Arthur, F.H.; Opit, G.P.; Zhu, K.Y.; Throne, J.E. Acute lethal and behavioral sublethal responses of two stored-product psocids to surface insecticides. Pest Manag. Sci. 2008, 64, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Arthur, F.H.; Throne, J.E. Residual efficacy of chlorfenapyr for control of stored-product psocids (Psocoptera). J. Econ. Entomol. 2014, 107, 854–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, J.; Doleckova-Maresova, L.; Hyblova, J.; Kudlikova, I.; Stejskal, V.; Mares, M. In vitro and in vivo inhibition of alpha-amylases of stored-product mite Acarus siro. Exp. Appl. Acarol. 2005, 35, 281–291. [Google Scholar] [CrossRef]
- Stara, J.; Stejskal, V.; Nesvorna, M.; Plachy, J.; Hubert, J. Efficacy of selected pesticides against synanthropic mites under laboratory assay. Pest Manag. Sci. 2011, 67, 446–457. [Google Scholar] [CrossRef]
- Stara, J.; Nesvorna, M.; Hubert, J. Comparison of the effect of insecticides on three strains of Tyrophagus putrescentiae (Acari: Astigmata) using an impregnated filter paper test and a growth test. Pest Manag. Sci. 2014, 70, 1138–1144. [Google Scholar] [CrossRef]
- Goddard, J. A crack-and-crevice application assay for selected insecticides to control the ham mite, Tyrophagus putrescentiae (Schrank). J. Entomol. Sci. 2017, 52, 304–307. [Google Scholar] [CrossRef]
- Hogsette, J.R.; Amendt, J. Flies. In Public Health Significance of Urban Pests. Bonnefoy, X., Kampen, H., Sweeney, K., Eds.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Stejskal, V.; Stara, J.; Pekar, S.; Nesvorna, M.; Hubert, J. Sensitivity of polyphagous (Plodia interpunctella) and stenophagous (Ephestia kuehniella) storage moths to residual insecticides: Effect of formulation and larval age. Insect Sci. 2020, 0, 1–11. [Google Scholar]
- Luckey, T.D. Insecticide hormoligosis. J. Econ. Entomol. 1968, 61, 7–12. [Google Scholar] [CrossRef]
- Abd-Elghafar, S.F.; Appel, A.G.; Mack, T.P. Toxicity of several insecticide formulations against adult German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 1990, 83, 2290–2294. [Google Scholar] [CrossRef]
- Jankov, D.; Indic, D.; Kljajic, P.; Almasi, R.; Andric, G.; Vukovic, S.; Grahovac, M. Initial and residual efficacy of insecticides on different surfaces against rice weevil Sitophilus oryzae (L.). J. Pest Sci. 2013, 86, 211–216. [Google Scholar] [CrossRef]
- Stejskal, V.; Aulicky, R. Human behaviour and application of residual insecticides to control storage and food industry pests. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Julius Kühn-Archiv: Berlin, Germany, 2010; pp. 916–918. [Google Scholar]
- Stejskal, V. Metapopulation concept and the persistence of urban pests in buildings. In Proceedings of the 4th International Conference on Urban Pests, Charleston, SC, USA, 7–10 July 2002; Jones, S.C., Zhai, J., Robinson, W.H., Eds.; Pocahontas Press: Blacksburg, VA, USA, 2002; pp. 75–85. [Google Scholar]
- Yao, J.; Chen, C.; Wu, H.; Chang, J.; Silver, K.; Campbell, J.F.; Arthur, F.K.; Zhu, K.Y. Differential susceptibilities of two closely-related stored product pests, the red flour beetle (Tribolium castaneum) and the confused flour beetle (Tribolium confusum), to five selected insecticides. J. Stored Prod. Res. 2019, 84, 101524. [Google Scholar] [CrossRef]
- Snoddy, E.T.; Appel, A.G. Field and laboratory efficacy of three insecticides for population management of the Asian cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2017, 107, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.K.; Reierson, D.A.; Hansgen, K.H. Control of American cockroaches (Dictyoptera: Blattidae) in sewers. J. Med. Entomol. 1991, 28, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.F.; Towes, M.D.; Arthur, F.H.; Arbogast, R.T. Long-term monitoring of Tribolium castaneum in two flour mills: Seasonal patterns and influence of fumigation. J. Econ. Entomol. 2010, 103, 991–1001. [Google Scholar] [CrossRef]
- Campbell, J.F.; Buckman, K.A.; Fields, P.G.; Subramanyam, B. Evaluation of structural treatment efficacy against Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) using meta-analysis of multiple studies conducted in food facilities. J. Econ. Entomol. 2015, 108, 2125–2140. [Google Scholar] [CrossRef] [Green Version]
- Doud, C.W.; Cuperus, G.W.; Kenkel, P.; Payton, M.E.; Phillips, T.W. Trapping Tribolium castaneum (Coleoptera: Tenebrionidae) and other beetles in flourmills: Evaluating fumigation efficacy and estimating population density. Insects 2021, 12, 144. [Google Scholar] [CrossRef]
- Toews, M.D.; Campbell, J.F.; Arthur, F.H.; West, M. Monitoring Tribolium castaneum (Coleoptera: Tenebrionidae) in pilot-scale warehouses treated with residual applications of (S)-hydroprene and cyfluthrin. J. Econ. Entomol. 2005, 98, 1391–1398. [Google Scholar] [CrossRef]
- Toews, M.D.; Arthur, F.H.; Campbell, J.F. Monitoring Tribolium castaneum (Herbst) in pilot-scale warehouses treated with b-cyfluthrin: Are residual insecticides and trapping compatible? Bull. Entomol. Res. 2009, 99, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Ebeling, W.; Reierson, D.A.; Wagner, R.E. Influence of repellency on the efficacy of blatticides. II. Laboratory experiments with German cockroaches. J. Econ. Entomol. 1967, 60, 1375–1390. [Google Scholar] [CrossRef]
- Schneider, B.M.; Bennett, G.W. Comparative studies of several methods for determining the repellency of blatticides. J. Econ. Entomol. 1985, 78, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Robinson, W.H. Pyrethroid resistance in a field population of German cockroach, Blatella germanica (L.). Jpn. A Sanit. Zool. 1991, 42, 241–244. [Google Scholar] [CrossRef]
- Gudrups, I.; Harris, A.H.; Dales, M.J. Are residual insecticide applications to store surfaces worth using? In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Wallingford, UK, 1994; pp. 785–789. [Google Scholar]
- Matthews, G.A. A History of Pesticides; CABI: Wallingford, UK, 2018. [Google Scholar]
- Mallis, A. Handbook of Pest Control, 10th ed.; Mallis Handbook LLC: Cleveland, OH, USA, 2011. [Google Scholar]
- Karner, M.; Price, R.G.; Roth, L.A. Laboratory evaluation of crack and crevice treatment for control of Blatella germanica by using various nozzle types, nozzle heights, and crack widths. J. Econ. Entomol. 1978, 71, 105–106. [Google Scholar] [CrossRef]
- Zungoli, P.A.; Robinson, W.H. Crack and crevice outshines fan spray. Pest Control 1982, 50, 20–22. [Google Scholar]
- Robinson, W.H.; Zungoli, P.A. Integrated control program for German cockroaches (Dictyoptera: Blattellidae) in multiple-unit dwellings. J. Econ. Entomol. 1985, 78, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Zungoli, P.A. Integrated pest management: An operational view. In Understanding and Controlling the German Cockroach; Rust, M.K., Owens, J.M., Reierson, D.A., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 345–359. [Google Scholar]
- Bonnefoy, X.; Kampen, H.; Sweeney, K. Public Health Significance of Urban Pests; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Zhai, J.; Robinson, W.H. Insecticide application technology: Chemical control of the German cockroach. Pest Control Tech. 1992, 40, 41–44. [Google Scholar]
- Zhai, J.; Robinson, W.H. Insecticide application technology: Methods and equipment for cockroach control. Pest Control Tech. 1992, 41, 78–84. [Google Scholar]
- Hewlett, P.S. The toxicities of three petroleum oils to the grain weevils. Ann. Appl. Biol. 1947, 34, 575–585. [Google Scholar] [CrossRef]
- Dove, W.E. Control of pests on animals and about milk products. J. Milk Food Technol. 1947, 10, 214–218. [Google Scholar] [CrossRef]
- Laing, M.D.; Gatarayiha, M.C.; Adandonon, A. Silicon use for pest control in agriculture: A review. Proc. S. Afr. Sugar Technol. Assoc. 2006, 80, 278–286. [Google Scholar]
- Li, B.X.; Liu, Y.; Zhang, P.; Li, X.X.; Pang, X.Y.; Zhao, Y.H.; Li, H.; Liu, F.; Lin, J.; Mu, W. Selection of organosilicone surfactants for tank-mixed pesticides considering the balance between synergistic effects on pests and environmental risks. Chemosphere 2019, 217, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Liszka, D.; Swietoslawski, P. Innovative formulations useful for area-wide application suitable for climate change. In Climate Change Impacts on Urban Pests; Dhang, P., Ed.; CABI: Wallingford, UK, 2017; Chapter 13; pp. 174–184. [Google Scholar]
- Baldwin, R.W.; Koehler, P.G.; Pereira, R.M. Toxicity of fatty acid salts to German and American cockroaches. J. Econ. Entomol. 2008, 101, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.R.; Balusu, R.R.; Ngumbi, E.N.; Appel, A.G. Topical and vapor toxicity of saturated fatty acids to the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2014, 107, 758–763. [Google Scholar] [CrossRef]
- Anonymous. Insecticides in Food Handling Establishments; Federal Register, No. 154; Environmenal Protection Agency (EPA): Los Angeles, CA, USA, 1973; Volume 38, pp. 21685–21686.
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop. Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Pinniger, D.P. A laboratory simulation of residual populations of stored product pests and an assessment of their susceptibility to a contact insecticide. J. Stored Prod. Res. 1974, 10, 217–223. [Google Scholar] [CrossRef]
- Barson, G. Laboratory assessment of the residual toxicity of commercial formulations of insecticides to adult Oryzaephilus surinamensis (Coleoptera: Silvidae) exposed for short time intervals. J. Stored Prod. Res. 1991, 27, 205–211. [Google Scholar] [CrossRef]
- Gould, F. Arthropod behaviour and the efficacy of plant protectant. Annu. Rev. Entomol. 1991, 36, 305–330. [Google Scholar] [CrossRef]
- Hodges, R.J.; Sidik, M.; Halid, H.; Conway, J.A. Cost efficiency of respraying store surfaces with insecticide to protect bagged milled rice from insect. Trop. Pest Manag. 1992, 38, 391–397. [Google Scholar] [CrossRef]
- Morrison, W.R.; Bruce, A.; Wilkins, R.V.; Albin, C.E.; Arthur, F.H. Sanitation improves stored product insect pest management. Insects 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Kucerova, Z.; Aulicky, R.; Stejskal, V. Accumulation of pest-arthropods in grain residues found in an empty store. J. Plant Dis. Prot. 2003, 110, 499–504. [Google Scholar] [CrossRef]
- Kilpatrick, J.W.; Schoof, H.F. The use of insecticide treated cords for housefly control. Public Health Rep. 1956, 71, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gostick, K.G.; Hewlett, P.S. Killing house-flies, Musca domestica L., by means of hanging drops of insecticide. Bull. Entomol. Res. 1960, 51, 523–532. [Google Scholar] [CrossRef]
- Tee, H.; Lee, C. Sustainable cockroach management using insecticidal baits: Formulations, behavioral responses and issues. In Urban Insect Pests-Sustainable Management Strategies; Dhang, P., Ed.; CAB International: Oxfordshire, UK; Boston, MA, USA, 2014; pp. 65–85. [Google Scholar]
- Markin, G.P.; OHill, S. Microencapsulated oil bait for control of the imported fire ant. J. Econ. Entomol. 1971, 64, 193–196. [Google Scholar] [CrossRef]
- Buczkowski, G.; Roper, E.; Chin, D. Polyacrylamide hydrogels: An effective tool for delivering liquid baits to pest ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2014, 107, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.W.; Choe, D.H.; Mulchandani, A.; Rust, M.K. Hydrogels: From controlled release to a new bait delivery for insect pest management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef]
- Klotz, J.H.; Shorey, H. Low-Toxic Control of Argentine Ants Using Pheromone-Enhanced Liquid Baits; California Department of Consumer Affairs: Sacramento, CA, USA, 2000; p. 35.
- Welzel, K.F.; Choe, D.H. Development of a pheromone-assisted baiting technique for Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2016, 109, 1303–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.M.; Smith, E.P. Quantifying the efficacy of an assessment-based pest management (APM) program for German Cockroach (L.) (Blattodea: Blattellidae) control in low-income public housing units. J. Econ. Entomol. 2020, 113, 375–384. [Google Scholar] [CrossRef]
- Buczkowski, G. The Trojan horse approach for managing invasive ants: A study with Asian needle ants, Pachycondyla chinensis. Biol. Invasions 2016, 18, 507–515. [Google Scholar] [CrossRef]
- Buczkowski, G.; Mothapo, N.P.; Wossler, T.C. Let them eat termites—prey-baiting provides effective control of Argentine ants, Linepithema humile, in a biodiversity hotspot. J. Appl. Entomol. 2018, 142, 504–512. [Google Scholar] [CrossRef]
- Miller, D.M.; Meek, F. Cost and efficacy comparison of integrated pest management strategies with monthly spray insecticide applications for German cockroach (Dictyoptera: Blattellidae) control in public housing. J. Econ. Entomol. 2004, 97, 559–569. [Google Scholar] [CrossRef]
- Wang, C.; Eiden, A.; Cooper, R.; Zha, C.; Wang, D.; Reilly, E. Changes in indoor insecticide residue levels after adopting an integrated pest management program to control German cockroach infestations in an apartment building. Insects 2019, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Mallis, A. Concentrations of sodium fluoride-flour mixtures for silverfish control. J. Econ. Entomol. 1944, 37, 842. [Google Scholar] [CrossRef]
- Sims, S.R.; Appel, A.G. Efficacy of commercial baits and new active ingredients against firebrats and silverfish (Zygentoma: Lepismatidae). J. Econ. Entomol. 2012, 105, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aak, A.; Hage, M.; Rukke, B.A. Long-tailed silverfish (Ctenolepisma longicaudata) control; bait choice based on primary and secondary poisoning. Insects 2020, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Aak, A.; Hage, M.; Lindstedt, H.H.; Rukke, B.A. Development of a poisoned bait strategy against the silverfish Ctenolepisma longicaudata (Escherich, 1905). Insects 2020, 11, 852. [Google Scholar] [CrossRef]
- Kucerova, Z.; Stejskal, V. Are commercial baits targeted on ants, silverfish and cockroaches applicable also for dometic psocids? First efficience report. In Proceedings of the 3rd International Conference on Urban Pests; Robinson, W.H., Ed.; Univ. of Agric.: Prague, Czech Republic, 1999; p. 638. [Google Scholar]
- Diaz-Montano, J.; Campbell, J.F.; Phillips, T.W.; Throne, J.E. Evaluation of potential attractants for six species of stored-product Psocids (Psocoptera: Liposcelididae, Trogiidae). J. Econ. Entomol. 2015, 108, 1398–1407. [Google Scholar] [CrossRef]
- Hall, A.V. Pest Control in Herbaria. Taxon 1988, 37, 885–907. [Google Scholar] [CrossRef]
- Cheng, T.H.; Campbell, F.L. Toxicity of phosphorous to cockroaches. J. Econ. Entomol. 1940, 33, 193–199. [Google Scholar] [CrossRef]
- Parker, C.; Baldwin, R.; Pereira, R.; Koehler, P. Evaluation of cyantraniliprole and other commercial fly baits under laboratory and field conditions. Insects 2015, 6, 977–987. [Google Scholar] [CrossRef] [Green Version]
- Appel, A.G. Laboratory and field performance of an indoxacarb bait against German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 2003, 96, 863–870. [Google Scholar] [CrossRef]
- Lovell, J.B. Amidinohydrazones-a new class of insecticides. In Proceedings of the 10th British Crop Protection Conference-Pests and Diseases, Brighton, UK, 19–22 November 1979; British Crop Protection Council: Cambridge, UK, 1979; pp. 575–582. [Google Scholar]
- Vander Meer, R.K.; Lofgren, C.S.; Williams, D.F. Fluoroaliphatic sulfones: A new class of delayed-action insecticides for control of Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 1985, 78, 1190–1197. [Google Scholar] [CrossRef]
- Appel, A.G.; Benson, E.P. Performance of abamectin bait formulations against German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 1995, 88, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lee, C.Y.; Rust, M.K. Biology and Management of the German Cockroach, 1st ed.; CABI: Wallingford, UK, 2021; pp. 1–304. [Google Scholar]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M.; Hughes, K.A.; Cordova, D.; Annan, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of cyantraniliprole a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorg. Med. Chem. Lett. 2013, 23, 6341–6345. [Google Scholar] [CrossRef]
- Lim, S.P.; Lee, C.Y. Effects of juvenile hormone analogs on new reproductives and colony growth of pharaoh ant (Hymenoptera: Formicidae). J. Econ. Entomol. 2005, 98, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, J.; Schmidt, J.; Bajomi, D. Occurrence of tropical and imported ant species in Europe (Hymenoptera: Formicidae). In Proceedings of the 6th International Conference on Urban Pests, Budapest, Hungary, 13–16 July 2008; Robinson, W.H., Bajomi, D., Eds.; OOK-Press Kft.: Pápai, Hungary, 2008. [Google Scholar]
- Kelany, Y.; Ibrahim, A.; Hegazy, M. Improving the efficiency of two local baits used for the control of the German cockroach, Blattella germanica (L.), (Dictyoptera: Blattellidae). Int. J. Pharm. Biol. Sci. 2017, 12, 59–66. [Google Scholar]
- Buczkowski, G.; Scherer, C.W.; Bennett, G.W. Horizontal transfer of bait in the German cockroach: Indoxacarb causes secondary and tertiary mortality. J. Econ. Entomol. 2008, 101, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V. Distribution of faeces of the German cockroach, Blattella germanica, in a new refuge. Entomol. Exp. Appl. 1997, 84, 201–205. [Google Scholar] [CrossRef]
- Silverman, J.; Vitale, G.L.; Shapas, T.J. Hydramethylnon uptake by Blattella germanica (Orthoptera: Blattellidae) by coprophagy. J. Econ. Entomol. 1991, 84, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopanic, R.J., Jr.; Schal, C. Coprophagy facilitates horizontal transmission of bait among cockroaches (Dictyoptera: Blattellidae). Environ. Entomol. 1999, 28, 431–438. [Google Scholar] [CrossRef]
- Buczkowski, G.; Schal, C. Method of insecticide delivery affects horizontal transfer of fipronil in the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2001, 94, 680–685. [Google Scholar] [CrossRef]
- Buczkowski, G.; Kopanic, R.J., Jr.; Schal, C. Vertical transfer and horizontal tranfers. J. Econ. Entomol. 2001, 94, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Lukas, J.; Aulicky, R. Speed of action of 10 commercial insecticidal gel-baits against the German cockroach, Blattella germanica. Int. Pest Control. 2004, 46, 185–189. [Google Scholar]
- Tay, J.W.; Lee, C.Y. Induced disturbances cause Monomorium pharaonis (Hymenoptera: Formicidae) nest relocation. J. Econ. Entomol. 2015, 108, 1237–1242. [Google Scholar] [CrossRef]
- Silverman, J.; Bieman, D.N. Glucose aversion in the German cockroach, Blattella germanica. J. Insect Physiol. 1993, 39, 925–933. [Google Scholar] [CrossRef]
- Hubbard, C.B.; Gerry, A.C. Genetic evaluation and characterization of behavioral resistance to imidacloprid in the house fly. Pestic. Biochem. Physiol. 2021, 171, 104741. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.C.; Ross, D.H.; Scott, J.G. Insecticide resistance monitoring of house fly populations from the United States. Pestic. Biochem. Physiol. 2019, 158, 61–68. [Google Scholar] [CrossRef]
- Mullens, B.A.; Gerry, A.C.; Diniz, A.N. Field and laboratory trials of a novel metaflumizone house fly (Diptera: Muscidae) bait in California. J. Econ. Entomol. 2010, 103, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.E.; Schal, C.; Silverman, J. Diet quality affects bait performance in German cockroaches (Dictyoptera: Blattellidae). Pest Manag. Sci. 2016, 72, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Wada-Katsumata, A.; Schal, C. Salivary digestion extends the range of sugar-aversions in the German cockroach. Insects 2021, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Molinero, F.; Arnau, J. Processing of dry-cured ham in a reduced-oxygen atmosphere: Effects on sensory traits. Meat Sci. 2010, 85, 420–427. [Google Scholar] [CrossRef] [PubMed]
- García, N. Efforts to control mites on Iberian ham by physical methods. Exp. Appl. Acarol. 2004, 32, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abbar, S.; Phillips, T.W.; Williams, J.B.; Smith, B.S.; Schilling, M.W. Developing food-grade coatings for dry-cured hams to protect against ham mite infestation. Meat Sci. 2016, 113, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Campbell, Y.; Shao, W.; Dinh, T.; To, K.; Rogers, W.; Zhang, X.; Phillips, T.; Schilling, W. Use of nets treated with food grade coatings on controlling mold growth and mite infestation in dry-cured ham aging facilities. J. Stored Prod. Res. 2020, 89, 101716. [Google Scholar] [CrossRef]
- Campbell, Y.L.; Zhang, X.; Shao, W.; Williams, J.B.; Kim, T.; Goddard, J.; Abbar, S.; Phillips, T.; Schilling, M.W. Use of nets treated with food-grade coatings on dry-cured ham to control Tyrophagus putrescentiae infestations without impacting sensory properties. J. Stored Prod. Res. 2018, 76, 30–36. [Google Scholar] [CrossRef]
- Hewlett, P.; Parkin, E. Effect of pretreatment on the toxicity of insecticidal films on building surfaces. Nature 1945, 155, 755–756. [Google Scholar] [CrossRef]
- Hewlett, P.S. The formation of insecticidal films on building materials. Ann. Appl. Biol. 1948, 35, 228–232. [Google Scholar] [CrossRef]
- Parkin, E.; Hewlett, S. The formation of insecticidal films on building materials. I. Preliminary experiments with films of pyrethrum and D.D.T. in a heavy oil. Ann. Appl. Biol. 1946, 33, 381. [Google Scholar] [CrossRef]
- Tyler, P.S.; Rowlands, D.G. Sodium carboxymethyl cellulose as a stabilizer for malathion formulations. J. Stored Prod. Res. 1967, 3, 109–115. [Google Scholar] [CrossRef]
- Cintora, C.; Barni, D. Pyrotechnic smoke generators as pesticide application tools. Int. Pest Control 2013, 55, 132–133. [Google Scholar]
- Deong, E.R.; Peer, K.C.; Fancher, L.W. A new generator for producing dry aerosols with organic insecticides. J. Econ. Entomol. 1950, 43, 542–546. [Google Scholar] [CrossRef]
- Marke, D.J.B.; Lilly, C.H. Smoke generators for the dispersion of pesticides. J. Sci. Food Agric. 1951, 2, 56–65. [Google Scholar] [CrossRef]
- Roff, M.W.; Griffiths, L.K.; Gobeau, N.; Johnson, P.D.; Pickering, D.; Rimmer, D.A.; Saunders, C.J.; Wheeler, J.P. Characteristics of pesticide pyrotechnic smoke devices. Ann. Occup. Hyg. 2006, 50, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jayas, D.S.; White, N.D.G.; Fields, P. Combined effect of carbon monoxide mixed with carbon dioxide in air on the mortality of stored-grain insects. J. Stored Prod. Res. 2009, 45, 247–253. [Google Scholar] [CrossRef]
- Sinha, S.N.; Singh, S.P.; Srivastava, C. Effect of smoke on Rhyzopertha dominica and Callosobruchus maculatus mortality and its susceptibility to phosphine. In Proceedings of the International Conference of Controlled Atmosphere and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; Donahaye, E.J., Ed.; Executive Printing Services: Clovis, NM, USA, 2001; pp. 431–437. [Google Scholar]
- Zerba, E. Fumigant canisters and other novel insecticide delivery systems for public health. Public Health Mag. 1995, 72, 62–68. [Google Scholar]
- Herford, G.V.B. The infestation of stored foodstuffs by insects. J. Sci. Food Agric. 1952, 3, 1–11. [Google Scholar] [CrossRef]
- Stejskal, V.; Aulicky, R.; Dohnal, P.; Kocourek, V.; Hajslova, J. Validation of insecticide aerosol generated by smoke-generator for German cockroach control. Int. Pest Control 2010, 52, 84–86. [Google Scholar]
- Krushelnycky, P. Evaluation of Water-Storing Granules as a Promising New Baiting Tool for the Control of Invasive Ants in Hawaii. Report of Year 1 Activities to the Hawaii Invasive Species Council. 2019. Available online: https://dlnr.hawaii.gov/hisc/files/2019/07/UH-CTAHR-KrushelnyckyP-Ant-Bait_FY18_Final-Report.pdf (accessed on 24 June 2021).
- Wagner, R.E.; Reierson, D.A. Yellow jacket control by baiting. 1. Influence of toxicants and attractants on bait acceptance. J. Econ. Entomol. 1969, 62, 1192–1197. [Google Scholar] [CrossRef]
- Chang, V. Toxic baiting of the Western yellowjacket (Hymenoptera: Vespidae) in Hawaii. J. Econ. Entomol. 1988, 81, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Spurr, E.B. Protein bait preferences of wasps (Vespula vulgaris and V. germanica) at Mt Thomas, Canterbury, New Zealand. N. Z. J Zool. 1995, 22, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Lee, L.C.; Na, J.; Loke, P.Y.; Lim, K.T.; Teo, E. Evaluation of methoprene granular baits against foraging Pharaoh ants, Monomorium pharaonis (Hymenoptera: Formicidae). Sociobiology 2003, 41, 717–723. [Google Scholar]
- Bajomi, D.; Lee, C.Y.; Lim, S.P.; Szilagyi, J.; Schmidt, J. Elimination of pharaoh’s ant, Monomorium pharaonis colonies with s-methoprene baits (Hymenoptera: Formicidae). In Proceedings of the Fifth International Conference on Urban Pests, Suntec, Singapore, 10–13 June 2005; Lee, C.Y., Robinson, W.H., Eds.; Perniagaan Ph’ng @ P&Y Design Network: Penang, Malaysia, 2005. [Google Scholar]
- Klunker, R.; Rupes, V.; Chmela, J. Control of Monomorium pharaonis using a methoprene bait in the Berlin Zoo and its combined application with a residue insecticide in the Olomouc Children’s Clinic. Angew. Parasitol. 1984, 25, 83–93. [Google Scholar]
- Eduku, A.; Maalekuu, B.; Kaledzi, P.; Tandoh, P.K. Development of bait for the management of coffee bean weevil, Araecerus fasciculatus in stored cocoa. Asian J. Agric. Res. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Vendl, T.; Frankova, M.; Aulicky, R.; Stejskal, V. First record of the development of Sitophilus oryzae on two rodent bait formulations and literature overview of stored product arthropods infestations in rodent baits. J. Stored Prod. Res. 2020, 86, 101557. [Google Scholar] [CrossRef]
- Brooke, M.D.L.; Cuthbert, R.J.; Harrison, G.; Gordon, C.; Taggart, M.A. Persistence of brodifacoum in cockroach and woodlice: Implications for secondary poisoning during rodent eradications. Ecotoxicol. Environ. Saf. 2013, 97, 183–188. [Google Scholar] [CrossRef]
- Lizzio, E.F. A boric acid-rodenticide mixture used in the control of coexisting rodent-cockroach infestations. Lab. Anim. Sci. 1986, 36, 74–76. [Google Scholar] [PubMed]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored product beetles. J. Stored Prod. Res. 2000, 36, e113–e118. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Vayias, B.J.; Tomanovic, Z.; Petrovic, A.; Rozman, V.; Adler, C.; Korunic, Z.; Milovanovic, D. Laboratory evaluation of diatomaceous earth deposits mined from several locations in central and south Eastern Europe as potential protectants against coleopteran grain pests. Crop Prot. 2011, 30, 329–339. [Google Scholar] [CrossRef]
- Headlee, T.J. Certain dusts as agents for the protection of stored seeds from insect infestation. J. Econ. Entomol. 1924, 17, 298–307. [Google Scholar] [CrossRef]
- Golob, P.; Webley, D.J. The Use of Plants and Minerals as Traditional Protectants of Stored Products; Tropical Product Institute: London, UK, 1980. [Google Scholar]
- Subramanyam, B.; Roesli, R. Inert dusts. In Alternatives to Pesticides in Stored-Product IPM; Springer: Boston, MA, USA, 2000; pp. 321–380. [Google Scholar]
- Zacher, F.; Kunike, G. Untersuchungen uber die Insektizide Wirkung von Oxyden und Karbonaten. Arb. Aus. Biol. Reichsans. 1931, 18, 201–231. [Google Scholar]
- Zacher, F. Neue Untersuchungen uber die Einwirkung Oberflachenaktiver Pulver auf Insekten. Zool. Anzeiger 1937, 10, 264–271. [Google Scholar]
- Parkin, E. Control of the granary weevil with finely ground mineral dusts. Ann. Appl. Biol. 1944, 31, 84–88. [Google Scholar] [CrossRef]
- David, W.A.L.; Gardiner, B.O.C. Factors influencing the action of dust insecticides. Bull. Entomol. Res. 1950, 41, 1–61. [Google Scholar] [CrossRef]
- Chiu, S.F. Toxicity studies of so-called inert materials with the bean weevil, Acanthoselides obtectus (Say.). J. Econ. Entomol. 1939, 32, 240–248. [Google Scholar] [CrossRef]
- Watkins, T.C.; Norton, L.B. A classification of insecticide dust diluents and carriers. J. Econ. Entomol. 1947, 40, 211–214. [Google Scholar] [CrossRef]
- Ebeling, W. Sorptive dust for pest control. Ann. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef] [PubMed]
- Zurek, L.; Gore, J.C.; Stringham, S.M.; Watson, D.W.; Waldvogel, M.G.; Schal, C. Boric acid dust as a component of an integrated cockroach management program in confined swine production. J. Econ. Entomol. 2003, 96, 1362–1366. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Elshaer, N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alif Alisha, A.S.; Thangapandiyan, S. Comparative bioassay of silver nanoparticles and malathion on infestation of red flour beetle Tribolium castaneum. JoBAZ 2019, 80, 1–10. [Google Scholar]
- Rahel, J.; Jonasova, E.; Nesvorna, M.; Klubal, R.; Erban, T.; Hubert, J. The toxic effect of chitosan/metal-impregnated textile to synanthropic mites. Pest Manag. Sci. 2013, 69, 722–7266. [Google Scholar] [CrossRef] [PubMed]
- Korunic, Z. Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Hamel, D.; Rozman, V.; Liska, A. Storage of cereals in warehouses with or without pesticides. Insects 2020, 11, 846. [Google Scholar] [CrossRef]
- Bohinc, T.; Horvat, A.; Andrić, G.; Golić, M.P.; Kljajić, P.; Trdan, S. Natural versus synthetic zeolites for controlling the maize weevil (Sitophilus zeamais)–like Messi versus Ronaldo? J. Stored Prod. Res. 2020, 88, 101639. [Google Scholar] [CrossRef]
- Li, Y.; Agarwal, M.; Cao, Y.; Ren, Y. Effect of synthetic amorphous silica powder on the cuticle of Tribolium castaneum and Sitophilus oryzae using hyperspectral imaging technique. Pest Manag. Sci. 2020, 76, 314–323. [Google Scholar] [CrossRef]
- Parkin, E.; Bills, G. Insecticidal dusts for the protection of stored peas and beans against bruchid infestation. Bull. Entomol. Res. 1955, 46, 625–641. [Google Scholar] [CrossRef]
- Wigglesworth, V. Action of inert dusts on insects. Nature 1944, 153, 493–494. [Google Scholar] [CrossRef]
- Korunic, Z.; Cenkowski, S.; Fields, P.G. Grain bulk density as affected by diatomaceous earth band application method. Postharvest Biol. Technol. 1998, 13, 81–89. [Google Scholar] [CrossRef]
- Perišić, V.; Vuković, S.; Perišić, V.; Pešić, S.; Vukajlović, F.; Andrić, G.; Kljajić, P. Insecticidal activity of three diatomaceous earths on lesser grain borer, Rhyzopertha dominica F., and their effects on wheat, barley, rye, oats and triticale grain properties. J. Stored Prod. Res. 2018, 75, 38–46. [Google Scholar] [CrossRef]
- Arthur, F.H. Evaluation of methoprence alone or in combination with diatomaceous earth to control Rhyzopertha dominica (Coleoptera: Bostrichidae) on stored wheat. J. Stored Prod. Res. 2004, 40, 485–498. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Vayias, B.J.; Kotzamanidis, S.; Synodis, S.D. Efficacy and adherence ratio of diatomaceous earth and spinosad in three wheat varieties against three stored-product insect pests. J. Stored Prod. Res. 2010, 46, 73–80. [Google Scholar] [CrossRef]
- Ceruti, F.C.; Lazzari, S.M.N. Combination of diatomaceous earth and powder deltamethrin for insect control in stored corn. Rev. Bras. Entomol. 2005, 49, 580–583. [Google Scholar] [CrossRef]
- Ziaee, M.; Ebadollahi, A.; Wakil, W. Integrating inert dusts with other technologies in stored productsprotection. Toxin Rev. 2019, 1–16. [Google Scholar] [CrossRef]
- Bridgeman, B.W. Structual Treatment Manual, an Instruction for the Application of Dryacide Slurry; GRAINCO Training Manual: Toowoomba, Australia, 1991. [Google Scholar]
- Desmarchelier, J.M.; Wright, E.J.; Allen, S.E. Dryacide®: A structural treatment for stored product insects. In Proceedings of the 5th Australian Applied Entomological Research Conference, Canberra, Australia, 28 April–1 May 1992; CSIRO Australia: Melbourne, Australia, 1992; pp. 483–485. [Google Scholar]
- Stejskal, V.; Kosina, P.; Kanyomeka, L. Arthropod pests and their natural enemies in stored crops in northern Namibia. J. Pest Sci. 2006, 79, 51–55. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Wang, P.; Wei, L.; Su, J. Efficacy of a Chinese diatomaceous earth and purpose-built sprayer for control of stored grain insect pests in an empty warehouse. In Proceedings of the 9th Int. Working Conference on Stored Product Prot., São Paulo, Brazil, 15–18 October 2006; pp. 849–854. [Google Scholar]
- Wilson, H.F.; Janes, R.J.; Campau, E.J. Electrostatic charge effects produced by insecticidal dusts. J. Econ. Entomol. 1944, 37, 651–655. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Vassilakos, T.N.; Dutton, A.C.; Jessop, N.; Sherwood, D.; Pease, G.; Brglez, A.; Storm, C.; Trdan, S. Combining electrostatic powder with an insecticide: Effect on stored-product beetles and on the commodity. Pest Manag. Sci. 2016, 72, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Gunther, F.A.; Lindgren, D.L.; Blinn, R.C. Biological effectiveness and persistence of malathion and lindane used for protection of stored wheat. J. Econ. Entomol. 1958, 51, 843–844. [Google Scholar] [CrossRef]
- Floyd, E.H. Effectiveness of malathion dust as a protectant for farm-stored corn in Louisiana. J. Econ. Entomol. 1961, 54, 900–904. [Google Scholar] [CrossRef]
- Wilbur, D.A. Effects of insecticidal dusts containing piperonyl butoxide and pyrethrins applied to wheat on the flavor of eggs. J. Econ. Entomol. 1952, 45, 899. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cao, Y.; Li, Y.; Yushu Gao, Y.; Feng, J. Food-grade inert dust as structural treatment against insect pests. In Proceedings of the 11th International Working Conference on Stored Product Prot., Chiang Mai, Thailand, 24–28 November 2014; Arthur, F.H., Kengkanpanich, R., Chayaprasert, W., Suthisut, D., Eds.; pp. 883–884. [Google Scholar] [CrossRef]
- Campolo, O.; Romeo, F.V.; Malacrinò, A.; Laudani, F.; Carpinteri, G.; Fabroni, S.; Rapisarda, P.; Palmeri, V. Effects of inert dusts applied alone and in combination with sweet orange essential oil against Rhyzopertha dominica (Coleoptera: Bostrichidae) and wheat microbial population. Ind. Crops Prod. 2014, 61, 361–369. [Google Scholar] [CrossRef]
- Korunic, Z.; Liska, A.; Lucic, P.; Hamel, D.; Rozman, V. Evaluation of diatomaceous earth formulations enhanced with natural products against stored product insects. J. Stored Prod. Res. 2020, 86, 101565. [Google Scholar] [CrossRef]
- Korunic, Z.; Fields, P.G.; Kovacs, M.I.P.; Noll, J.S.; Lukow, O.M.; Demianyk, C.J.; Shibley, K.J. The effect of diatomaceous earth on grain quality. Postharvest Biol. Technol. 1996, 9, 373–387. [Google Scholar] [CrossRef]
- Arthur, F.H. Residual efficacy of a deltamethrin emulsifiable concentrate formulation against Rhyzopertha dominica (F.) and Sitotroga cerealella (Oliver) after partial treatment of brown rice. Insects 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Vardeman, E.A.; Arthur, F.H.; Nechols, J.R.; Campbell, J.F. Effect of temperature, exposure internal and depth of diatomaceous earth on distribution, mortality, and reproduction of the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) in stored wheat. J. Econ. Entomol. 2006, 99, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Dales, M.J. A Review of Plant Materials Used for Controlling Insect Pests of Stored Products (NRI Bulletin 65); Natural Resources Institute: Chatham, UK, 1996. [Google Scholar]
- Bohinc, T.; Horvat, A.; Andrić, G.; Golić, M.P.; Kljajić, P.; Trdan, S. Comparison of three different wood ashes and diatomaceous earth in controlling the maize weevil under laboratory conditions. J. Stored Prod. Res. 2018, 79, 1–8. [Google Scholar] [CrossRef]
- Mehta, V.; Kumar, S. Influence of different plant powders as grain protectants on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in stored wheat. J. Food Prot. 2020, 83, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Haq, T.; Usmani, N.F.; Abbas, T. Screening of plant leaves as grain protectants against Tribolium castaneum during storage. Pak. J. Bot. 2005, 37, 149–153. [Google Scholar]
- Goudoungou, J.W.; Nukenine, E.N.; Suh, C.; Gangué, T.; Ndjonka, D. Effectiveness of binary combinations of Plectranthus glandulosus leaf powder and Hymenocardia acida wood ash against Sitophilus zeamais (Coleoptera: Curculionidae). Agric. Food Secur. 2018, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Derbalah, A.; Ahmed, S. Oil and powder of spearmint as an alternative to Sitophilus oryzae chemical control of wheat grains. J. Plant Prot. Res. 2011, 51, 1–6. [Google Scholar] [CrossRef]
- Nandi, R.; Naganagoud, A.; Patil, B.V. Effect of sweet flag rhizome, Acorus calamus L. formulations with cow dung ash as a carrier against Callasobruchus chinensis Linn. in pigeonpea. Karnataka J. Agric. Sci. 2008, 21, 45–48. [Google Scholar]
- Kathirvelu, C.; Muthukumaran, N.; Kanagarajan, R.; Mangayarkarasi, S. Fumigant effect of tablet formulation of certain botanicals against key pests of stored produce in laboratory conditions. J. Appl. Sci. Comput. 2019, 6, 914–923. [Google Scholar]
- Kathirvelu, C. Evaluation of phyto tablet formulation against key insect pests of stored produce under laboratory conditions. J. Pharmacogn. Phytochem. 2019, SP2, 387–390. [Google Scholar]
- Chang, Y.; Lee, S.H.; Na, J.H.; Chang, P.S.; Han, J. Protection of grain products from Sitophilus oryzae (L.) contamination by anti-insect pest repellent sachet containing allyl mercaptan microcapsule. J. Food. Sci. 2017, 82, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Zdarkova, E.; Horak, E.; Pulpan, J. Chemical and Non-Chemical Methods of Control of Mites Infesting Stored Seeds (Research Report- in Czech); Research Institute of Food Industry: Prague, Czech Republic, 1974; p. 117. [Google Scholar]
- Stejskal, V.; Vendl, T.; Frankova, M.; Aulicky, R. Overview of stored product rodents, insects and mites associated with beet and beet products. (Přehled skladištních hlodavců, hmyzu a roztočů škodících na semenech cukrové řepy a řepných produktech.) Listy Cukrov. Řepař. 2019, 135, 248–254. [Google Scholar]
- Hosseini, H.; Hamgini, E.Y.; Jafari, S.M.; Bolourian, S. Improving the oxidative stability of sunflower seed kernels by edible biopolymeric coatings loaded with rosemary extract. J. Stored Prod. Res. 2020, 89, 101729. [Google Scholar] [CrossRef]
- Watters, F.L. Protection of packaged food from insect infestation by use of silica gel. J. Econ. Entomol. 1966, 59, 146–149. [Google Scholar] [CrossRef]
- Scheff, D.S.; Arthur, F.H.; Myers, S.W. Evaluation of methoprene-treated packaging against Trogoderma granarium Everts and Trogoderma inclusum LeConte larval development and packaging penetration or invasion. J. Stored Prod. Res. 2019, 84, 101530. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H. Effectiveness of insecticide-incorporated bags to control stored-product beetles. J. Stored Prod. Res. 2017, 70, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Marsin, A.M.; Muhamad, I.I.; Anis, S.N.S.; Lazim, N.A.M.; Ching, L.W.; Dolhaji, N.H. Essential oils as insect repellent agents in food packaging: A review. Eur. Food Res. Technol. 2020, 246, 1519–1532. [Google Scholar] [CrossRef]
- Navarro, S.; Zehavi, D.; Angel, S.; Finkelman, S. Natural nontoxic insect repellent packaging materials. In Intelligent and Active Packaging for Fruits and Vegetables; Wilson, C.L., Ed.; CRC Press: New York, NY, USA, 2007; pp. 201–236. [Google Scholar]
- Licciardello, F.; Muratore, G.; Suma, P.; Russo, A.; Nerín, C. Effectiveness of a novel insect-repellent food packaging incorporating essential oils against the red flour beetle (Tribolium castaneum). Innov. Food Sci. Emerg. Technol. 2013, 19, 173–180. [Google Scholar] [CrossRef]
- Wilkins, R.V.; Zhu, K.Y.; Campbell, J.F.; Morrison, W.R., III. Mobility and dispersal of two cosmopolitan stored-product insects are adversely affected by long-lasting insecticide netting in a life stage-dependent manner. J. Econ. Entomol. 2020, 113, 1768–1779. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Sakka, M.; Schaffert, S.; Sterz, T.; Austin, J.W.; Bozoglou, C.; Klitsinaris, P.; Athanassiou, C.G. Evaluation of Carifend®, an alpha-cypermethrin-coated polyester net, for the control of Lasioderma serricorne and Ephestia elutella in stored tobacco. J. Pest Sci. 2018, 91, 751–759. [Google Scholar] [CrossRef]
- Agrafioti, P.; Faliagka, S.; Lampiri, E.; Orth, M.; Pätzel, M.; Katsoulas, N.; Athanassiou, C.G. Evaluation of silica-coated insect proof nets for the control of Aphis fabae, Sitophilus oryzae, and Tribolium confusum. Nanomaterials 2020, 10, 1658. [Google Scholar] [CrossRef] [PubMed]
- Paloukas, Y.Z.; Agrafioti, P.; Rumbos, C.I.; Schaffert, S.; Sterz, T.; Bozoglou, C.; Klitsinaris, P.; Austin, J.W.; Athanassiou, C.G. Evaluation of Carifend® for the control of stored-product beetles. J. Stored Prod. Res. 2020, 85, 101534. [Google Scholar] [CrossRef]
- Morrison III, W.R.; Wilkins, R.V.; Gerken, A.R.; Scheff, D.S.; Zhu, K.Y.; Arthur, F.H.; Campbell, J.F. Mobility of adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after exposure to long-lasting insecticide-incorporated netting. J. Econ. Entomol. 2018, 111, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, R.; Snetselaar, J.; Suer, R.A.; Osinga, A.J.; Deschietere, J.; Lyimo, I.N.; Mnyone, L.L.; Brooke, B.D.; Ranson, H.; Knols, B.G.J.; et al. Electrostatic coating breaks mosquito resistance. Proc. Nat. Acad. Sci. USA 2015, 112, 12081–12086. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, T.M.; Subramanyam, B.; Zhu, K.Y.; Campbell, J.F. Contact toxicity of filter cake and triplex powders from Ethiopia against adults of Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 1469–1475. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V.; Frydova, B. Field validation of phosphine efficacy on the first recorded resistant strains of Sitophilus granaries and Tribolium castaneum from the Czech Republic. J. Stored Prod. Res. 2019, 81, 107–113. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 1–12. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Savoldelli, S.; Trematerra, P. Mass-trapping, mating-disruption and attracticide methods for managing stored-product insects: Success stories and research needs. Stewart Postharvest Rev. 2011, 7, 1–8. [Google Scholar]
- Morrison, W.R., III; Scully, E.D.; Campbell, J.F. Towards developing areawide semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag. Sci. 2021, 77, 2667–2682. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Athanassiou, C.G.; Schilling, M.W.; Phillips, T.W. Biology and management of the red-legged ham beetle, Necrobia rufipes DeGeer (Coleoptera: Cleridae). J. Stored Prod. Res. 2020, 88, 106135. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Athanassiou, C.G. Use of entomopathogenic fungi for the control of stored-product insects: Can fungi protect durable commodities? J. Pest Sci. 2017, 90, 839–854. [Google Scholar] [CrossRef]
- Chikhanis, G.G.; Sakka, M.K.; Athanassiou, C.G. Efficacy of Beauveria bassiana in combination with an electrostatically charged dust for the control of major stored-product beetle species on concrete. J. Stored Prod. Res. 2018, 79, 139–143. [Google Scholar]
- Wakil, W.; Schmitt, T.; Kavallieratos, N.G. Mortality and progeny production of four stored-product insect species on three grain commodities treated with Beauveria bassiana and diatomaceous earths. J. Stored Prod. Res. 2020, 101738. [Google Scholar] [CrossRef]
- McFarlane, D.J.; Aitken, E.A.B.; Ridley, A.W.; Walter, G.H. The dietary relationships of Tribolium castaneum (Herbst) with microfungi. J. Appl. Entomol. 2021, 145, 158–169. [Google Scholar] [CrossRef]






| Category of Insecticide Formulation and Methods for Delivery to Target | Subcategory (Type) of the Insecticide Application Formulation |
|---|---|
| Vapors and gases | Vaporization and sublimation (cold, thermal, “residual fumigation“, etc.) |
| Toxic gases (released from solid, liquid, or gas formulations) | |
| Inert gases (hypoxic/anoxic atmospheres) | |
| Liquids | Admixture, dressing, dipping, and impregnation treatments of grain |
| Coatings, paintings, and lacquers (structural surface treatments) | |
| Liquid baits | |
| Liquid droplets in air delivered as space treatment by aerosols, thermal or cold/ULV/fogs or mists | |
| Insecticide deposits on surface delivered as spray (indirect residual treatment) | |
| Direct treatment of arthropods by sprays | |
| Foams, gels, and pastes | Expandable foams as insecticide barriers and cavity fillers |
| Foam baits | |
| Gel, hydrogel, and paste baits | |
| Protective food gel coatings and layers on meat and cheese | |
| Solids | Smokes (solid aerosols) |
| Solid baits | |
| Dust, slurries, powders, ash, and nanoparticles | |
| Insecticides incorporated into protective nets | |
| Insecticide impregnation of packages (incorporation into the matrix/surface coating) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects 2021, 12, 590. https://doi.org/10.3390/insects12070590
Stejskal V, Vendl T, Aulicky R, Athanassiou C. Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects. 2021; 12(7):590. https://doi.org/10.3390/insects12070590
Chicago/Turabian StyleStejskal, Vaclav, Tomas Vendl, Radek Aulicky, and Christos Athanassiou. 2021. "Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control" Insects 12, no. 7: 590. https://doi.org/10.3390/insects12070590
APA StyleStejskal, V., Vendl, T., Aulicky, R., & Athanassiou, C. (2021). Synthetic and Natural Insecticides: Gas, Liquid, Gel and Solid Formulations for Stored-Product and Food-Industry Pest Control. Insects, 12(7), 590. https://doi.org/10.3390/insects12070590