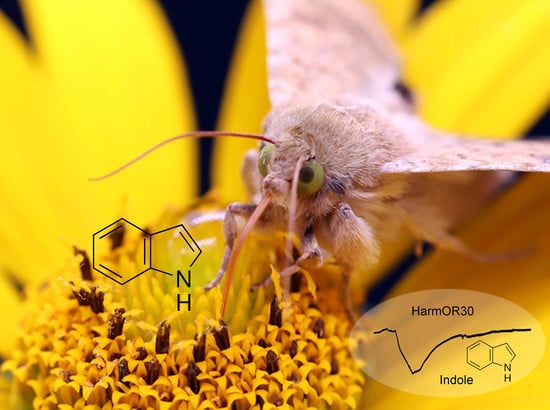
An Odorant Receptor from the Proboscis of the Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) Narrowly Tuned to Indole
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Extraction, cDNA Synthesis, and HarmORs Cloning
2.3. Sequences Alignment of HarmORs and Transmembrane Domains Analysis
2.4. Heterologous Expression of HarmORs: cRNA Synthesis and Oocyte Microinjection
2.5. Plant Volatile Compounds for Ligand Screening of HarmORs
2.6. Whole-Cell Two-Electrode Voltage-Clamp Physiological Recording
2.7. Phylogenetic Analysis of Lepidopteran ORs
3. Results
3.1. Full-Length Cloning of HarmOR Genes from the Proboscis
3.2. Functional Identification of HarmORs
3.3. Functional Comparison of the HarmOR30 Orthologs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Krenn, H.W. (Ed.) Insect Mouthparts: Form, Function, Development and Performance; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 5, pp. 1–8. [Google Scholar]
- Chapman, R. Mouthparts and feeding. In The Insects Structure and Function, 5th ed.; Cambridge Univeristy Press: Cambridge, UK, 2013; pp. 15–22. [Google Scholar]
- Haverkamp, A.; Bing, J.; Badeke, E.; Hansson, B.S.; Knaden, M. Innate olfactory preferences for flowers matching proboscis length ensure optimal energy gain in a hawkmoth. Nat. Commun. 2016, 7, 11644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenn, H.W. (Ed.) Fluid-Feeding Mouthparts. In Insect Mouthparts: Form, Function, Development and Performance; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 47–99. [Google Scholar]
- Krenn, H.W. Functional morphology and movements of the proboscis of Lepidoptera (Insecta). Zoomorphology 1990, 110, 105–114. [Google Scholar] [CrossRef]
- Bauder, J.A.S.; Karolyi, F. Superlong Proboscises as Co-adaptations to Flowers. In Insect Mouthparts: Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 479–527. [Google Scholar]
- Kwon, H.W.; Lu, T.; Rützler, M.; Zwiebel, L.J. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2006, 103, 13526–13531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haverkamp, A.; Yon, F.; Keesey, I.W.; Mißbach, C.; Koenig, C.; Hansson, B.S.; Baldwin, I.T.; Knaden, M.; Kessler, D. Hawkmoths evaluate scenting flowers with the tip of their proboscis. eLife 2016, 5, e15039. [Google Scholar] [CrossRef] [Green Version]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life. Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M. Molecular Evolution of the Major Arthropod Chemoreceptor Gene Families. Annu. Rev. Entomol. 2019, 64, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Benton, R. Molecular mechanisms of olfactory detection in insects: Beyond receptors. Open. Biol. 2020, 10, 200252. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.E.; Raming, K.; Breer, H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.B.; Roy, A.; Anderson, P.; Schlyter, F.; Hansson, B.S.; Larsson, M.C. Transcriptome analysis of gene families involved in chemosensory function in Spodoptera littoralis (Lepidoptera: Noctuidae). BMC Genom. 2019, 20, 428. [Google Scholar] [CrossRef]
- Du, L.; Zhao, X.; Liang, X.; Gao, X.; Liu, Y.; Wang, G. Identification of candidate chemosensory genes in Mythimna separata by transcriptomic analysis. BMC Genom. 2018, 19, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gu, S.; Zhang, Y.; Guo, Y.; Wang, G. Candidate olfaction genes identified within the Helicoverpa armigera Antennal Transcriptome. PLoS ONE 2012, 7, e48260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, B.; Dong, S.; Cao, D.; Dong, J.; Walker, W.B.; Liu, Y.; Wang, G. Antennal transcriptome analysis and comparison of chemosensory gene families in two closely related noctuidae moths, Helicoverpa armigera and H. assulta. PLoS ONE 2015, 10, e0117054. [Google Scholar] [CrossRef]
- Guo, M.; Chen, Q.; Liu, Y.; Wang, G.; Han, Z. Chemoreception of Mouthparts: Sensilla Morphology and Discovery of Chemosensory Genes in Proboscis and Labial Palps of Adult Helicoverpa armigera (Lepidoptera: Noctuidae). Front. Physiol. 2018, 9, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Du, L.; Chen, Q.; Feng, Y.; Zhang, J.; Zhang, X.; Tian, K.; Cao, S.; Huang, T.; Jacquin-Joly, E.; et al. Odorant Receptors for Detecting Flowering Plant Cues Are Functionally Conserved across Moths and Butterflies. Mol. Biol. Evol. 2021, 38, 1413–1427. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, K.; Wu, Z.; Xu, J.; Erb, M. Plant volatiles as regulators of plant defense and herbivore immunity: Molecular mechanisms and unanswered questions. Curr. Opin. Insect. Sci. 2021, 44, 82–88. [Google Scholar] [CrossRef]
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity—origins, specificity, perception and signaling. Curr. Opin. Plant. Biol. 2018, 44, 117–121. [Google Scholar] [CrossRef]
- de Fouchier, A.; Walker, W.B.; Montagné, N.; Steiner, C.; Binyameen, M.; Schlyter, F.; Chertemps, T.; Maria, A.; François, M.-C.; Monsempes, C.; et al. Functional evolution of Lepidoptera olfactory receptors revealed by deorphanization of a moth repertoire. Nat. Commun. 2017, 8, 15709. [Google Scholar] [CrossRef]
- Knudsen, J.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Crippen, T.L.; Wu, G.; Griffin, A.S.; Wood, T.K.; Kilner, R.M. Indole: An evolutionarily conserved influencer of behavior across kingdoms. BioEssays 2017, 39, 1600203. [Google Scholar] [CrossRef]
- Huigens, M.E.; Woelke, J.B.; Pashalidou, F.G.; Bukovinszky, T.; Smid, H.M.; Fatouros, N.E. Chemical espionage on species-specific butterfly anti-aphrodisiacs by hitchhiking Trichogramma wasps. Behav. Ecol. 2010, 21, 470–478. [Google Scholar] [CrossRef]
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Handrick, V.; Robert, C.A.M.; Ahern, K.R.; Zhou, S.; Machado, R.A.R.; Maag, D.; Glauser, G.; Fernandez-Penny, F.E.; Chandran, J.N.; Rodgers-Melnik, E.; et al. Biosynthesis of 8-O-Methylated Benzoxazinoid Defense Compounds in Maize. Plant Cell 2016, 28, 1682–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, M.; Chomet, P.; Glawischnig, E.; Stettner, C.; Grün, S.; Winklmair, A.; Eisenreich, W.; Bacher, A.; Meeley, R.B.; Briggs, S.P.; et al. Analysis of a Chemical Plant Defense Mechanism in Grasses. Science 1997, 277, 696–699. [Google Scholar] [CrossRef]
- Frey, M.; Stettner, C.; Paré, P.W.; Schmelz, E.A.; Tumlinson, J.H.; Gierl, A. An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 2000, 97, 14801–14806. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Fiesselmann, A.; Zhao, N.; Chen, H.; Frey, M.; Chen, F. Biosynthesis and emission of insect herbivory-induced volatile indole in rice. Phytochemistry 2012, 73, 15–22. [Google Scholar] [CrossRef]
- Frey, M.; Spiteller, D.; Boland, W.; Gierl, A. Transcriptional activation of Igl, the gene for indole formation in Zea mays: A structure–activity study with elicitor-active N-acyl glutamines from insects. Phytochemistry 2004, 65, 1047–1055. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, M.; Held, M.; Triponez, Y.; Turlings, T.C.J. The Role of Indole and Other Shikimic Acid Derived Maize Volatiles in the Attraction of Two Parasitic Wasps. J. Chem. Ecol. 2006, 32, 2733–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veyrat, N.; Robert, C.A.M.; Turlings, T.C.J.; Erb, M. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol. 2016, 104, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Veyrat, N.; Xu, H.; Hu, L.; Turlings, T.C.J.; Erb, M. An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 2018, 4, eaar4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turlings, T.C.J.; Lengwiler, U.B.; Bernasconi, M.L.; Wechsler, D. Timing of induced volatile emissions in maize seedlings. Planta 1998, 207, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Ye, M.; Erb, M. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant Cell Environ. 2019, 42, 959–971. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Glauser, G.; Lou, Y.; Erb, M.; Hu, L. Molecular Dissection of Early Defense Signaling Underlying Volatile-Mediated Defense Regulation and Herbivore Resistance in Rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Liu, M.; Erb, M.; Glauser, G.; Zhang, J.; Li, X.; Sun, X. Indole primes defence signalling and increases herbivore resistance in tea plants. Plant Cell Environ. 2021, 44, 1165–1177. [Google Scholar] [CrossRef]
- Andersson, J.; Borg-Karlson, A.-K.; Wiklund, C. Antiaphrodisiacs in Pierid Butterflies: A Theme with Variation! J. Chem. Ecol. 2003, 29, 1489–1499. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Pashalidou, F.G.; Aponte Cordero, W.V.; van Loon, J.J.A.; Mumm, R.; Dicke, M.; Hilker, M.; Huigens, M.E. Anti-aphrodisiac Compounds of Male Butterflies Increase the Risk of Egg Parasitoid Attack by Inducing Plant Synomone Production. J. Chem. Ecol. 2009, 35, 1373–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef]
- Chandravadana, M.V.; Srinivas, M.; Murthy, N. Indole in Tuberose (Polianthes tuberosa) Varieties. J. Essent. Oil Res. 1994, 6, 653–655. [Google Scholar] [CrossRef]
- Zhou, H.C.; Hou, Z.W.; Wang, D.X.; Ning, J.M.; Wei, S. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac flowers. Food Chem. 2019, 286, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Jürgens, A.; Campbell, D.R. Floral scent in natural hybrids of Ipomopsis (Polemoniaceae) and their parental species. Ann. Bot. Lond. 2013, 113, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, M.; Raguso, R.A.; Jürgens, A.; Campbell, D.R. Context-dependent reproductive isolation mediated by floral scent and color. Evolution 2015, 69, 1–13. [Google Scholar] [CrossRef]
- Yan, Z.G.; Wang, C.Z. Similar attractiveness of maize volatiles induced by Helicoverpa armigera and Pseudaletia separata to the generalist parasitoid Campoletis chlorideae. Entomol. Exp. Appl. 2006, 118, 87–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Wang, M.; Yu, W.; Wyckhuys, K.A.G.; Wu, K. Floral Visitation Can Enhance Fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) Long-Distance Migrants. J. Econ. Entomol. 2019, 112, 2655–2662. [Google Scholar] [CrossRef]
- Li, R.T.; Huang, L.Q.; Dong, J.F.; Wang, C.Z. A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles. eLife 2020, 9, e53706. [Google Scholar] [CrossRef]
- Euler, M.; Baldwin, I.T. The chemistry of defense and apparency in the corollas of Nicotiana attenuata. Oecologia 1996, 107, 102–112. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin Ian, T. Field Experiments with Transformed Plants Reveal the Sense of Floral Scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef] [Green Version]
- Kessler, D.; Kallenbach, M.; Diezel, C.; Rothe, E.; Murdock, M.; Baldwin, I.T. How scent and nectar influence floral antagonists and mutualists. eLife 2015, 4, e07641. [Google Scholar] [CrossRef]
- Heinrich, B.; Raven, P.H. Energetics and Pollination Ecology. Science 1972, 176, 597–602. [Google Scholar] [CrossRef]



| Gene Name | Primers for Full-Length Cloning (5′-3′) | |
|---|---|---|
| HarmOrco | Forward | ATGATGACCAAGGTGAAGGCCCAGG |
| Reverse | TTATTTGAGTTGTACCAACACCATG | |
| HarmOR24 | Forward | ATGGATTCCAAAATGTCGCTGTC |
| Reverse | CTACTTTGTCTGCCGAAGAACC | |
| HarmOR30 | Forward | ATGTTTTCTTCGGAAGATTTGT |
| Reverse | TTATGTCGTCTGATTCAACACTGC | |
| HarmOR58 | Forward | ATGGACGTCCCTTCGTTGAAAGA |
| Reverse | TTAATACATAACTGCAAAGAAAGAGT | |
| Primers for constructing expression vector * (5′-3′) | ||
| HarmOR24 | Forward | ATCACTAGTGGGCCCgccaccATGGATTCCAAAATGTCGCTGTC |
| Reverse | CTAGTCAGTCGCGGCCGCCTACTTTGTCTGCCGAAGAACC | |
| HarmOR30 | Forward | ATCACTAGTGGGCCCgccaccATGTTTTCTTCGGAAGATTTGTTTC |
| Reverse | CTAGTCAGTCGCGGCCGCTTATGTCGTCTGATTCAACACTGCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Ren, X.; Liu, Y.; Wang, G. An Odorant Receptor from the Proboscis of the Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) Narrowly Tuned to Indole. Insects 2022, 13, 385. https://doi.org/10.3390/insects13040385
Guo M, Ren X, Liu Y, Wang G. An Odorant Receptor from the Proboscis of the Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) Narrowly Tuned to Indole. Insects. 2022; 13(4):385. https://doi.org/10.3390/insects13040385
Chicago/Turabian StyleGuo, Mengbo, Xueting Ren, Yang Liu, and Guirong Wang. 2022. "An Odorant Receptor from the Proboscis of the Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) Narrowly Tuned to Indole" Insects 13, no. 4: 385. https://doi.org/10.3390/insects13040385
APA StyleGuo, M., Ren, X., Liu, Y., & Wang, G. (2022). An Odorant Receptor from the Proboscis of the Cotton Bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) Narrowly Tuned to Indole. Insects, 13(4), 385. https://doi.org/10.3390/insects13040385