Survey of Natural Enemies of the Invasive Boxwood Moth Cydalima perspectalis in Southwestern Mediterranean Europe and Biocontrol Potential of a Native Beauveria bassiana (Bals.-Criv.) Vuill. Strain
Abstract
:Simple Summary
Abstract
1. Introduction
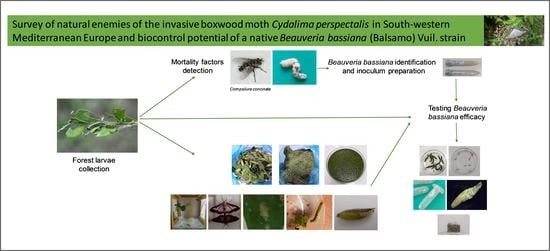
2. Materials and Methods
2.1. Insect Rearing
2.2. Semiartificial Diet
2.3. Detection of Natural Factors of Mortality
2.4. Fungal Strain and Inoculum Preparation
2.5. Efficacy of Beauveria bassiana in Controlling Cydalima Perspectalis Larvae
2.5.1. Bioassay with Sixth Instar (L6) Field-Collected Larvae
2.5.2. Bioassay with L2–L3 and L4–L5 Laboratory-Reared Larvae
2.6. Statistical Analysis
3. Results
3.1. Presence of Parasitoids
3.2. Identification of Beauveria bassiana Strain
3.3. Efficacy of Beauveria bassiana in Controlling Cydalima Perspectalis Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krüger, E.O. Glyphodes perspectalis (Walker, 1859) neu für die Fauna Europas (Lepidoptera: Crambidae). Entomol. Z. Insektenboerse 2008, 118, 81–83. [Google Scholar]
- EPPO. 2018. Available online: https://gd.eppo.int/taxon/DPHNPE/reporting (accessed on 1 June 2022).
- López, C.; Eizaguirre, M. Diapause and biological cycle of Cydalima perspectalis in the eastern Pyrenees. J. Appl. Entomol. 2019, 143, 1096–1104. [Google Scholar] [CrossRef]
- Wainhouse, D. Plant health. In Ecological Methods in Forest Pest Management; Oxford University Press: Oxford, UK; New York, NY, USA, 2005; ISBN 198505647 (hbk). [Google Scholar]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, B.J.M.; Linder, S.; Fanning, P.D.; Isaacs, R.; Szücs, M. Experimental adaptation of native parasitoids to the invasive insect pest, Drosophila suzukii. Biol. Control 2022, 167, 104843. [Google Scholar] [CrossRef]
- Albajes, R.; Alomar, O. Current and potential use of polyphagous predators. In Integrated Pest Management in Greehouse Crops, Developments in Plant Pathology; Albajes, R., Gullino, L.M., van Lenteren, J.C., Elad, Y., Eds.; Springer: Dordrecht, The Neatherlands, 1999; Volume 14, pp. 265–275. [Google Scholar]
- González-Mas, N.; Ortega-García, L.; Garrido-Jurado, I.; Dembilio, O.; Jaques, J.A.; Quesada-Moraga, E. Which came first: The disease or the pest? Is there a host mediated spread of Beauveria bassiana (Ascomycota: Hypocreales) by invasive palm pests? J. Invertebr. Pathol. 2019, 162, 26–42. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Macdonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by Exotic Forest Pests: A Threat to Forest Ecosystems. For. Sci. Monogr. 1995, 30, 49. [Google Scholar] [CrossRef]
- Lupi, D. A 3-year field survey of the natural enemies of the horse-chestnut leaf miner Cameraria ohridella in Lombardy, Italy. BioControl 2005, 50, 113–126. [Google Scholar] [CrossRef]
- Zappalá, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arno, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Crisol Martínez, E.; Van der Blom, J. Necremnus tutae is widespread and efficiently controls Tuta absoluta in tomato greenhouses in S-E Spain. IOBC-WPRS Bull. 2019, 147, 22–29. [Google Scholar]
- Pérez-Otero, R.; Mansilla, J.P.; Vidal, M. Cydalima perspectalis Walker, 1859 (Lepidoptera, Crambidae): Una nueva amenaza para Buxus spp. en la Península Ibérica. Arq. Entomolóxicos 2014, 10, 225–228. [Google Scholar]
- Matošević, D. Box Tree Moth (Cydalima perspectalis, Lepidoptera; Crambidae), New Invasive Insect Pest in Croatia. South-East Eur. For. 2013, 4, 89–94. [Google Scholar] [CrossRef]
- Tuske, E.; Marczali, Z. Study of the seasonal flight of the box tree moth (Cydalima prespectalis Walker 1859) in county Zala, Hungary in 2015. Novenyvedelem 2016, 52, 65–68. [Google Scholar]
- Oltean, I.; Ulujan, I.; Hulujan, I.; Varga, M.; Totos, S.; Florian, T. Cydalima perspectalis walker (Lepidoptera, Crambidae) a new dangerous pest report on Buxus Sempervirens in Cluj Area. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Agric. 2017, 74, 26–36. [Google Scholar] [CrossRef]
- Santi, F.; Radeghieri, P.; Inga Sigurta, G.; Maini, S. Sex pheromone traps for detection of the invasive box tree moth in Italy. Bull. Insectol. 2015, 68, 158–160. [Google Scholar]
- Venard, M.; Formez, N.; Rocher, F.; Colombel, E.; Baubet, O.; Corréard, M.; Tabone, E. Quelle régulation de la pyrale du buis en milieu natural? Phytoma 2019, 723, 28–33. [Google Scholar]
- Ferracini, C.; Pogolotti, C.; Mancardi, P.; Miglio, M.; Bonelli, S.; Barbero, F. The Box Tree Moth: An Invasive Species Severely Threatening Buxus Natural Formation in NW Italy. Forests 2022, 13, 178. [Google Scholar] [CrossRef]
- Las Heras, S.; Arimany, M.; Artola, J.; Bassols, E. Desarrollo de métodos para una gestión integrada de la polilla del boj (Cydalima perspectalis) (Lepidoptera: Crambidae) en parques, jardines y espacios verdes. Phytoma 2019, 308, 56–62. [Google Scholar]
- Bird, S.; Raper, C.; Dale-Skey, N.; Salisbury, A. First records of two natural enemies of box tree moth, Cydalima perspectalis (Lepidoptera: Crambidae), in Britain. Br. J. Entomol. Nat. Hist. 2020, 33, 67–70. [Google Scholar]
- Zamani, S.M.; Farahani, S.; Farashiani, M.E.; Salehi, M.; Samavat, S. The first record of Beauveria bassiana on box tree moth, Cydalima perspectalis in Iran. Iran. J. Forest Range Protect Res. 2017, 15, 199–202. [Google Scholar]
- Ghavamabad, R.G.; Talebi, A.A.; Mehrabadi, M.; Farashiani, M.E.; Pedram, M. First record of Oscheius myriophilus (Poinar, 1986) (Rhabditida: Rhabditidae) from Iran; and its efficacy against two economic forest trees pests, Cydalima perspectalis (Walker, 1859) (Lepidoptera: Crambidae) and Hyphantria cunea (Drury, 1773) (Lepidoptera: Erebidae) in laboratory condition. J. Nematol. 2021, 53, 1–16. [Google Scholar] [CrossRef]
- Peterlin, A.; Rodic, K.B.E.; Trdan, S. The occurrence of the box tree moth (Cydalima perspectalis) in southeastern Slovenia in [Conference poster]. Pojav puspanove vesce (Cydalima perspectalis) na obmocju jugovzhodne Slovenije v letu 2014. In Proceedings of the Zbornik Predavanj in Referatov 12. Slovenskega Posvetovanja o Varstvu Rastlin z Mednarodno Udelezbo, Ptuj, Slovenija, 3–4 March 2015; pp. 314–317. [Google Scholar]
- SangMyeong, L.; DongWoon, L.; HoYul, C.; JiWoong, P. Pathogenicities of Beauveria bassiana GY1-17 against some agro-forest insect pests. Korean J. Appl. Entomol. 1997, 36, 351–356. [Google Scholar]
- Las Heras, S.; Arimany, M. Desenvolupament de Mètode s Per Monitoritzar i Controlar Cydalima Perspectalis (Lepidoptera: Crambidae) Plaga Exòtica Defoliadora de Boixos, a Parcs i Jardins. Associació de Professionals dels Espais Verds de Catalunya: Barcelona, Spain, 2020; p. 21p. [Google Scholar]
- Oberemok, V.V.; Laikova, K.V.; Shumskykh, M.N.; Zaitsev, A.S. The first record of box tree moth in Crimea and a novel perspective of its biological control based on Lymantria dispar multicapsid nuclear polyhedrosis virus and DNA insecticides approach. Entomol. Gen. 2017, 36, 207–217. [Google Scholar] [CrossRef]
- Rose, J.; Jehle, J.A.; Kleespies, R.G. Biological control of the box tree moth Cydalima perspectalis with Anagrapha falcifera nucleopolyhedrovirus (AnfaNPV). IOBC/WPRS Bull. 2013, 90, 169–172. [Google Scholar]
- Goettig, S.; Herz, A. Susceptibility of the Box tree pyralid Cydalima perspectalis Walker (Lepidoptera: Crambidae) to potential biological control agents Neem (NeemAzal®-T/S) and entomopathogenic nematodes (Nemastar®) assessed in laboratory bioassays and field trials. J. Plant Dis. Protect. 2018, 125, 365–375. [Google Scholar] [CrossRef]
- Miladinović, Z.; Mitrić, S.; Jakšić, B.; Nježić, B. Evaluation of potential of four entomopathogenic nematodes to control box tree moth (Cydalima perspectalis Walker). In Proceedings of the XI International Symposium on Agricultural Sciences AgroReS, Trebinje, Bosnia and Herzegovina, 26–28 May 2022; pp. 158–165. [Google Scholar]
- Gokturk, T.; Chachkhiani-Anasashvili, N.; Kordali, S.; Dumbadze, G.; Bozhuyuk , A.U. Insecticidal effects of some essential oils against box tree moth Cydalima perspectalis Walker (Lepidoptera: Crambidae)). Int. J. Trop. Insect. Sci. 2021, 41, 313–322. [Google Scholar] [CrossRef]
- Szelényi, M.O.; Erdei, A.L.; Jósvai, J.K.; Radványi, D.; Sümegi, B.; Vétek, G.; Molnár, B.P.; Kárpáti, Z. Essential oil headspace volatiles prevent invasive box tree moth (Cydalima perspectalis) Oviposition insights from electrophysiology and behaviour. Insects 2020, 11, 465. [Google Scholar] [CrossRef]
- Kárpáti, Z.; Molnár, B.P. Volatile compounds of larval excrete repel box tree moth (Cydalima perspectalis) oviposition. FT A puszpangmoly (Cydalima perspectalis) larvak urulekenek illata gatolja a fajtars nostenyek tojasrakasat. Novenyvedelem 2017, 53, 241–248. [Google Scholar]
- Molnár, B.P.; Tóth, Z.; Kárpáti, Z. Synthetic blend of larval frass volatiles repel oviposition in the invasive box tree moth, Cydalima perspectalis. J. Pest Sci. 2017, 90, 873–885. [Google Scholar] [CrossRef]
- Goettig, S.; Herz, A. Are egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) promising biological control agents for regulating the invasive Box tree pyralid, Cydalima perspectalis (Lepidoptera: Crambidae)? Biocontrol Sci. Technol. 2016, 26, 1471–1488. [Google Scholar] [CrossRef]
- Bras, A.; Auger-Rozenberg, M.A.; Pineau, P.; Lorme, P.; Roques, A.; Laparie, M.; Rousselet, J. Effect of emamectin benzoate tree micro injection on the survival rate of the first larval instar of the box tree moth [Conference poster]. FT Effet de micro-injections d’emamectine benzoate dans l’arbre hote sur la survie des premiers stades larvaires de la pyrale du buis. In Proceedings of the 6e COMAPPI, Conference sur les Moyens Alternatifs de Protection Pour une Production Integree, Lille, France, 21–23 March 2017. [Google Scholar]
- Fora, C.G.; Sasu, L.; Posta, D.; Berar, C. Chemical possibilities of Cydalima perspectalis Walk. (Lepidoptera: Crambidae) control. J. Hort. Forest Biotechnol. 2016, 20, 3–34. [Google Scholar]
- Qian, X.L.; Xue, W.D.; Wei, N.; Chang, L. Occurrence rule and control test of Diaphania perspectalis Walker in Rugao City. J Jiangsu Forest Sci. Technol. 2018, 452, 38–41. [Google Scholar]
- Barnett, L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; MacMillan Publishing: New York, NY, USA, 1987; p. 218. [Google Scholar]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 153–185. [Google Scholar]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.N.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Marquez, M.; Ortiz-Urquiza, A.; Santiago-Alvarez, C.; Iturriaga, E.A.; Quesada-Moraga, E.; Monte, E.; Hermosa, R. Genetic analyses place most Spanish isolates of Beauveria bassiana in a molecular group with wordwide distribution. BMC Microbiol. 2011, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Statgraphics. Statgraphics Plus; Version 3.0; Manugistics: Rockville, MD, USA, 1997. [Google Scholar]
- Tachi, T.; Huang, Y.Z.; Komagata, S.; Araya, K.; Dawood, M.M.; Pham, T.H.; Yang, D.; Zeegers, T.; Tschorsnig, H.-P.; Shima, H. Systematic study of the genus Compsilura Bouché in Southeast and East Asia with morphological and molecular data (Diptera, Tachinidae). J. Asia Pac. Entomol. 2021, 24, 285–296. [Google Scholar] [CrossRef]
- Morel, E.; Capelli, M.; de Bodard, M.; Colombel, E.; Michel, T.; Tabone, E. Research for native parasites and predators of the box tree moth Cydalima perspectalis, in natural boxwood forest in France. In Proceedings of the International Scientific Events—10th International Conference Agriculture & Food, Burgas, Bulgaria, 16–19 August 2021; pp. 231–242, ⟨hal-03420290⟩. [Google Scholar]
- Farahani, S.; Salehi, M.; Farashiani, M.E.; Gilasian, E.; Terujeni, S.N.; Ahangaran, Y. Compsilura concinnata (Meigen), parasitoid of Box tree moth, Cydalima perspectalis (Walker) from Iran. Iran. J. Forest Range Protect. Res. 2018, 16, 102–106. [Google Scholar] [CrossRef]
- Hulujan, I.B.; Florian, T.; Florian, V.C.; Oltean, I. Zoophagous entomofauna and entomopathogenic agents reported on Cydalima perspectalis (Walker, 1859) in north-western of Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 11786. [Google Scholar] [CrossRef]
- Martini, A.; Di Vitantonio, C.; Dindo, M.L. Acceptance and suitability of the box tree moth Cydalima perspectalis as host for the tachinid parasitoid Exorista larvarum. Bull. Insectol. 2019, 72, 150–160. [Google Scholar]
- Agenjo, R. Monografia de la familia Thaumetopoidae. Rev. Eos 1941, 17, 69–130. [Google Scholar]
- Cerretti, P.F.; Tschorsnig, H.-P. Annotated host catalogue for the Tachinidae (Diptera) of Italy. Stuttg. Beitr. Nat. A 2010, 3, 305–340. [Google Scholar]
- Webber, T.; Schaffner, J.V., Jr. Host Relations of Compsilura Concinnata Meigen, an Important Tachinid Parasite of the Gipsy Moth and the Brown-Tail Moth; United States Department of Agriculture, Department Bulletin: Wasington, DC, USA, 1926; p. 32.
- Harizanova, V.; Naydenov, M.; Stoeva, A.; Valcheva, I.; Draganova, D.; Borisov, Y.; Mohamedova, M. Survey of the gut pathogenic microflora associated with caterpillars of the box tree moth Cydalima perspectalis Walker, 1859 (Lepidoptera: Crambidae). Acta Entomol. Serbica 2018, 23, 15–25. [Google Scholar] [CrossRef]
- Burjanadze, M.; Supatashvili, A.; Göktürk, T. Control strategies against invasive pest box tree moth Cydalima perspectalis in Georgia. SETSCI Conf. Index. Syst. 2019, 4, 1–4. [Google Scholar]
- Kim, J.J.; Roberts, D.W. The relationship between conidial dose, moulting and insect develop-mental stage on the susceptibility of cotton aphid, Aphis gossypii, to conidia of Lecanicillium attenuatum, an ento-mopathogenic fungus. Biocontrol Sci. Technol. 2012, 22, 319–331. [Google Scholar] [CrossRef]



| Components | % of Total Weight |
|---|---|
| Water | 78.76 |
| Agar–agar | 1.8 |
| Freeze-dried leaves of Buxus sempervirens | 19 |
| Benzoic acid | 0.3 |
| Nipagin | 0.1 |
| Aureomycin | 0.04 |
| Year | Area | Coordinates | Number of Larvae |
|---|---|---|---|
| 2018 | Garrotxa (Olot) | 42°10′16.80″ N 2°28′51.84″ E | 100 |
| 2019 | Garrotxa (Olot) | 42°10′21.36″ N 2°28′1.32″ E | 150 |
| 2020 | Ripolles (Ribes de Freser) | 42°17′59.82″ N 2° 9′52.88″ E | 150 |
| 2021 | Ripolles (Fustanyà) | 42°20′23.30″ N 2°10′35.60″ E | 100 |
| Moianes (Sta Maria d’Oló) | 41°52′4.76″ N 2° 1′33.72″ E | 100 | |
| 2022 | Solsones (Solsona) | 41°55′35.87″ N 1°29′42.28″ E | 200 |
| Year | Area | % Parasitism | Number of Dead Larvae |
|---|---|---|---|
| 2018 | Garrotxa (Olot) | 0 | 6 |
| 2019 | Garrotxa (Olot) | 0 | 8 |
| 2020 | Ripolles (Ribes de Freser) | 20 | 5 |
| 2021 | Ripolles (Fustanyà) | 0.5 | 12 |
| Moianes (Sta Maria d’Oló) | 1 | 6 | |
| 2022 | Solsones (Solsona) | 0.5 | 5 |
| Larval Instar | Logrank Test | Wilcoxon Test | ||
|---|---|---|---|---|
| χ2 | p | χ2 | p | |
| L2–L3 | 14.87 | <0.001 | 13.78 | <0.001 |
| L4–L5 | 16.93 | <0.001 | 17.05 | <0.001 |
| L6 field | 110.28 | <0.001 | 97.67 | <0.001 |
| L2–L3 | L4–L5 | |
|---|---|---|
| Number of larvae producing a cocoon | 2.39 ± 0.28 (18) a | 3.83 ± 0.28 (18) b |
| Total number of live larvae | 7.22 ± 0.33 (18) | 8.61 ± 0.33 (18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.; Las Heras, S.; Garrido-Jurado, I.; Quesada-Moraga, E.; Eizaguirre, M. Survey of Natural Enemies of the Invasive Boxwood Moth Cydalima perspectalis in Southwestern Mediterranean Europe and Biocontrol Potential of a Native Beauveria bassiana (Bals.-Criv.) Vuill. Strain. Insects 2022, 13, 781. https://doi.org/10.3390/insects13090781
López C, Las Heras S, Garrido-Jurado I, Quesada-Moraga E, Eizaguirre M. Survey of Natural Enemies of the Invasive Boxwood Moth Cydalima perspectalis in Southwestern Mediterranean Europe and Biocontrol Potential of a Native Beauveria bassiana (Bals.-Criv.) Vuill. Strain. Insects. 2022; 13(9):781. https://doi.org/10.3390/insects13090781
Chicago/Turabian StyleLópez, Carmen, Sandra Las Heras, Inmaculada Garrido-Jurado, Enrique Quesada-Moraga, and Matilde Eizaguirre. 2022. "Survey of Natural Enemies of the Invasive Boxwood Moth Cydalima perspectalis in Southwestern Mediterranean Europe and Biocontrol Potential of a Native Beauveria bassiana (Bals.-Criv.) Vuill. Strain" Insects 13, no. 9: 781. https://doi.org/10.3390/insects13090781
APA StyleLópez, C., Las Heras, S., Garrido-Jurado, I., Quesada-Moraga, E., & Eizaguirre, M. (2022). Survey of Natural Enemies of the Invasive Boxwood Moth Cydalima perspectalis in Southwestern Mediterranean Europe and Biocontrol Potential of a Native Beauveria bassiana (Bals.-Criv.) Vuill. Strain. Insects, 13(9), 781. https://doi.org/10.3390/insects13090781