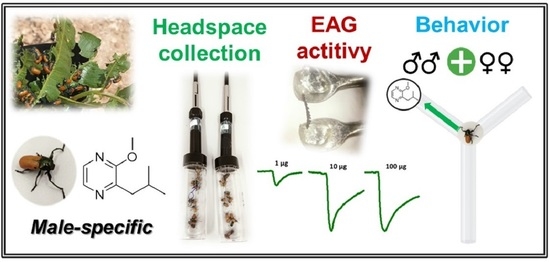
Laboratory Evidence of 2-Isobutyl-3-methoxypyrazine as a Male-Released Aggregative Cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Headspace Solid-Phase Microextraction (HS-SPME)
2.4. Electroantennographic (EAG) Response
2.5. Behavioral Bioassays
2.6. Statistical Analysis
3. Results
3.1. HS-SPME Collections
3.2. EAG Response
3.3. Behavioral Bioassays
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehouse, W.E. The pistachio nut—A new crop for the Western United States. Econ. Bot. 1957, 11, 281–321. [Google Scholar] [CrossRef]
- Lemaistre, J.C. Le pistachier (étude bibliographique). Fruits 1959, 14, 57–77. [Google Scholar]
- Maggs, D.H. An Introduction to Pistachio Growing in Australia; Commonwealth Scientific and Industrial Research Organization: Adelaide, Australia, 1982; ISBN 0643029907. [Google Scholar]
- Mehlenbacher, S.A. Progress and prospects in nut breeding. Acta Hortic. 2003, 622, 57–79. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio nuts (Pistacia vera L.): Production, nutrients, bioactives and novel health effects. Plants 2021, 11, 18. [Google Scholar] [CrossRef]
- Mourikis, P.A.; Tsourgianni, A.; Chitzanidis, A. Pistachio nut insect pests and means of control in Greece. Acta Hortic. 1998, 470, 604–611. [Google Scholar] [CrossRef]
- Mehrnejad, M.R. Arthropod pests of pistachios, their natural enemies and management. Plant Prot. Sci. 2020, 56, 231–260. [Google Scholar] [CrossRef]
- MAPAMA. Guía de Gestión Integrada de Plagas—Pistacho; MAPAMA: Madrid, Spain, 2018; ISBN 9788449114984.
- Davatchi, G.A. Biology of the insect fauna of cultivated and wild Pistacia. Rev. Pathol. Veg. 1958, 37, 3–166. [Google Scholar]
- Bolu, H. Investigations on the fauna of insects and mites in pistachio areas in south Eastern Anatolia region of Turkey. Turkish J. Entomol. 2002, 26, 197–208. [Google Scholar]
- Doğanlar, M.; Karadağ, S.; Mendel, Z. Notes on pistachio seed wasps from two locations in the east Mediterranean. Phytoparasitica 2009, 37, 147–151. [Google Scholar] [CrossRef]
- Rice, R.E.; Michailides, T.J. Pistachio seed chalcid, Megastigmus pistaciae Walker (Hymenoptera: Torymidae), in California. J. Econ. Entomol. 1988, 81, 1446–1449. [Google Scholar] [CrossRef]
- Braham, M.; Jardak, T. Contribution a l’etude de la bio-ecologie du scolyte du pistachier Chaetoptelius vestitus Muls & Rey (Coleoptera, Scolytidae) dans les regions du centre et du sud tunisiens. Rev. Ezzaitouna 2012, 13, 1–17. [Google Scholar]
- Mehrnejad, M.R. Investigation into the overwintering and winter-management of the common pistachio psyllid, Agonoscena pistaciae (Hemiptera: Aphalaridae), a major pest in pistachio plantations. Zool. Ecol. 2018, 28, 384–388. [Google Scholar] [CrossRef]
- Haviland, D.R.; Bentley, W.J.; Siegel, J.P.; Holtz, B.A.; Daane, K.M.; Higbee, B.S. Navel orangeworm and obliquebanded leafroller. In Pistachio Production Manual, Publication 3545; Ferguson, L., Haviland, D.R., Eds.; University of California, Agriculture and Natural Resources: Oakland, CA, USA, 2016; pp. 197–210. [Google Scholar]
- Fernández-Carrillo, E.; Fernández-Carrillo, J.L.; Rodrigo-Gómez, S.; Velázquez-de-Castro, A.J. Weevils (Coleoptera, Curculionidae) associated with pistachio tree (Pistacia vera L.) crops in Castilla-La Mancha (Central Spain). Graellsia 2022, 78, e168. [Google Scholar] [CrossRef]
- Gómez, S.R.; Gil-Tapetado, D.; García-Gila, J.; Blasco-Aróstegui, J.; Polidori, C. The leaf beetle Labidostomis lusitanica (Coleoptera: Chrysomelidae) as an Iberian pistachio pest: Projecting risky areas. Pest Manag. Sci. 2022, 78, 217–229. [Google Scholar] [CrossRef]
- Heisswolf, A.; Gabler, D.; Obermaier, E.; Müller, C. Olfactory versus contact cues in host plant recognition of a monophagous chrysomelid beetle. J. Insect Behav. 2007, 20, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Gerritsma, D.A.; Brindle, I.D.; Jones, T.R.B.; Capretta, A. Preparation of labelled 2-methoxy-3-alkylpyrazines: Synthesis and characterization of deuterated 2-methoxy-3-isopropylyrazine and 2-methoxy-3-isobutylpyrazine. J. Label. Compd. Radiopharm. 2003, 46, 243–253. [Google Scholar] [CrossRef]
- Bohman, B.; Phillips, R.D.; Menz, M.H.M.; Berntsson, B.W.; Flematti, G.R.; Barrow, R.A.; Dixon, K.W.; Peakall, R. Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: Implications for the evolution of sexual deception. New Phytol. 2014, 203, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Bohman, B.; Peakall, R. Pyrazines attract Catocheilus thynnine wasps. Insects 2014, 5, 474–487. [Google Scholar] [CrossRef]
- Maia, A.C.D.; Santos, G.K.N.; Gonçalves, E.G.; do Amaral Ferraz Navarro, D.M.; Nuñez-Avellaneda, L.A. 2-Alkyl-3-methoxypyrazines are potent attractants of florivorous scarabs (Melolonthidae, Cyclocephalini) associated with economically exploitable Neotropical palms (Arecaceae). Pest Manag. Sci. 2018, 74, 2053–2058. [Google Scholar] [CrossRef]
- Suinyuy, T.N.; Donaldson, J.S.; Johnson, S.D. Geographical matching of volatile signals and pollinator olfactory responses in a cycad brood-site mutualism. Proc. R. Soc. B Biol. Sci. 2015, 282, 20152053. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.P.; Brown, W.V.; Rothschild, M. Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology 1990, 1, 43–51. [Google Scholar] [CrossRef]
- Burdfield-Steel, E.; Pakkanen, H.; Rojas, B.; Galarza, J.A.; Mappes, J. De novo synthesis of chemical defenses in an aposematic moth. J. Insect Sci. 2018, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, M.; Moore, B.P.; Brown, W.V. Pyrazines as warning odour components in the Monarch butterfly, Danaus plexippus, and in moths of the genera Zygaena and Amata (Lepidoptera). Biol. J. Linn. Soc. 1984, 23, 375–380. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Avery, J.W.; Lee, C.-J.; Graf, J.C.; Harrison, D.J.; Bin, F. Semiochemistry of cabbage bugs (Heteroptera: Pentatomidae: Eurydema and Murgantia). J. Entomol. Sci. 1996, 31, 172–182. [Google Scholar] [CrossRef]
- Rowe, C.; Guilford, T. Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature 1996, 383, 520–522. [Google Scholar] [CrossRef]
- Lindström, L.; Rowe, C.; Guilford, T. Pyrazine odour makes visually conspicuous prey aversive. Proc. Biol. Sci. 2001, 268, 159–162. [Google Scholar] [CrossRef]
- Rojas, B.; Burdfield-Steel, E.; Pakkanen, H.; Suisto, K.; Maczka, M.; Schulz, S.; Mappes, J. How to fight multiple enemies: Target-specific chemical defences in an aposematic moth. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171424. [Google Scholar] [CrossRef]
- Burdfield-Steel, E.R.; Schneider, J.M.; Mappes, J.; Dobler, S. Testing the effectiveness of pyrazine defences against spiders. Chemoecology 2020, 30, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Al Abassi, S.; Birkett, M.A.; Pettersson, J.; Pickett, J.A.; Woodcock, C.M. Ladybird beetle odour identified and found to be responsible. Cell. Mol. Life Sci. 1998, 54, 876–879. [Google Scholar] [CrossRef]
- Wheeler, C.A.; Cardé, R.T. Defensive allomones function as aggregation pheromones in diapausing ladybird beetles, Hippodamia convergens. J. Chem. Ecol. 2013, 39, 723–732. [Google Scholar] [CrossRef]
- Santiago-Blay, J.; Jolivet, P.; Verma, K. A natural history of conspecific aggregations in terrestrial arthropods, with emphasis on cycloalexy in leaf beetles (Coleoptera: Chrysomelidae). Terr. Arthropod Rev. 2012, 5, 289–355. [Google Scholar] [CrossRef] [Green Version]
- Dickens, J.C.; Oliver, J.E.; Hollister, B.; Davis, J.C.; Klun, J.A. Breaking a paradigm: Male-produced aggregation pheromone for the Colorado potato beetle. J. Exp. Biol. 2002, 205, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Jiménez-Alemán, G.H.; Lin, M.-Y.; Hsu, Y.-C.; Mewis, I.; Srinivasan, R.; Ulrichs, C.; Boland, W.; Hansson, B.S.; Reinecke, A. The aggregation pheromone of Phyllotreta striolata (Coleoptera: Chrysomelidae) revisited. J. Chem. Ecol. 2016, 42, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W.; Bean, D.W.; Petroski, R.J. The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): Identification of two behaviorally active components. J. Chem. Ecol. 2005, 31, 657–670. [Google Scholar] [CrossRef]
- Tansey, J.A.; McClay, A.S.; Cole, D.E.; Keddie, B.A. Evidence for the influence of conspecific chemical cues on Aphthona nigriscutis (Coleoptera: Chrysomelidae) behaviour and distribution. BioControl 2005, 50, 343–358. [Google Scholar] [CrossRef]
- Zilkowski, B.W.; Bartelt, R.J.; Cossé, A.A.; Petroski, R.J. Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula): Identification, synthesis, and field biossays. J. Chem. Ecol. 2006, 32, 2543–2558. [Google Scholar] [CrossRef]
- Rao, S.; Cossé, A.A.; Zilkowski, B.W.; Bartelt, R.J. Aggregation pheromone of the cereal leaf beetle: Field evaluation and emission from males in the laboratory. J. Chem. Ecol. 2003, 29, 2165–2175. [Google Scholar] [CrossRef]
- Beran, F.; Mewis, I.; Srinivasan, R.; Svoboda, J.; Vial, C.; Mosimann, H.; Boland, W.; Büttner, C.; Ulrichs, C.; Hansson, B.S.; et al. Male Phyllotreta striolata (F.) produce an aggregation pheromone: Identification of male-specific compounds and interaction with host plant volatiles. J. Chem. Ecol. 2011, 37, 85–97. [Google Scholar] [CrossRef]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W. Identification and electrophysiological activity of a novel hydroxy ketone emitted by male cereal leaf beetles. J. Nat. Prod. 2002, 65, 1156–1160. [Google Scholar] [CrossRef]
- Smyth, R.R.; Hoffmann, M.P. A male-produced aggregation pheromone facilitating Acalymma vittatum [F.] (Coleoptera: Chrysomelidae) early-season host plant colonization. J. Insect Behav. 2003, 16, 347–359. [Google Scholar] [CrossRef]
- Peng, C.; Bartelt, R.J.; Weiss, M.J. Male crucifer flea beetles produce an aggregation pheromone. Physiol. Entomol. 1999, 24, 98–99. [Google Scholar] [CrossRef] [Green Version]
- Vander Meer, R.K.; Preston, C.A.; Choi, M.-Y. Isolation of a pyrazine alarm pheromone component from the fire ant, Solenopsis invicta. J. Chem. Ecol. 2010, 36, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Morgan, E.D.; Oldham, N.J.; Liebig, J. Recruitment pheromone in the harvester ant genus Pogonomyrmex. J. Insect Physiol. 2001, 47, 369–374. [Google Scholar] [CrossRef]
- Tentschert, J.; Bestmann, H.-J.; Hölldobler, B.; Heinze, J. 2,3-Dimethyl-5-(2-methylpropyl)pyrazine, a trail pheromone component of Eutetramorium mocquerysi Emery (1899) (Hymenoptera: Formicidae). Naturwissenschaften 2000, 87, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Chuman, T.; Landolt, P.J.; Heath, R.R.; Tumlinson, J.H. Isolation, identification, and synthesis of male-produced sex pheromone of papaya fruit fly, Toxotrypana curvicauda Gerstaecker (Diptera: Tephritidae). J. Chem. Ecol. 1987, 13, 1979–1992. [Google Scholar] [CrossRef]
- Robledo, N.; Vega, M.; Escalante, J.; Arzuffi, R. A new component of the male papaya fruit fly (Diptera: Tephritidae) sex pheromone. Fla. Entomol. 2014, 97, 1260–1262. [Google Scholar] [CrossRef]
- Robacker, D.; Aluja, M.; Bartelt, R.J.; Patt, J. Identification of chemicals emitted by calling males of the sapote fruit fly, Anastrepha serpentina. J. Chem. Ecol. 2009, 35, 601–609. [Google Scholar] [CrossRef]
- Susset, E.C.; Ramon-Portugal, F.; Hemptinne, J.-L.; Dewhirst, S.Y.; Birkett, M.A.; Magro, A. The role of semiochemicals in short-range location of aggregation sites in Adalia bipunctata (Coleoptera, Coccinellidae). J. Chem. Ecol. 2013, 39, 591–601. [Google Scholar] [CrossRef]
- Cudjoe, E.; Wiederkehr, T.B.; Brindle, I.D. Headspace gas chromatography-mass spectrometry: A fast approach to the identification and determination of 2-alkyl-3-methoxypyrazine pheromones in ladybugs. Analyst 2005, 130, 152–155. [Google Scholar] [CrossRef]
- Gaffke, A.M.; Sing, S.E.; Millar, J.G.; Dudley, T.L.; Bean, D.W.; Peterson, R.K.D.; Weaver, D.K. An herbivore-induced plant volatile from saltcedar (Tamarix spp.) is repellent to Diorhabda carinulata (Coleoptera: Chrysomelidae). Environ. Entomol. 2020, 49, 1063–1070. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Kotwica, K.; Łyszczarz, A.; Delaney, K.J. Gastrophysa polygoni herbivory on Rumex confertus: Single leaf VOC induction and dose dependent herbivore attraction/repellence to individual compounds. J. Plant Physiol. 2011, 168, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Wenda-Piesik, A.; Krasińska, A.; Wrzesińska, D.; Delaney, K.J. Volatile organic compounds released by Rumex confertus following Hypera rumicis herbivory and weevil responses to volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Wiedenmann, R.N.; Raghu, S. Early-summer pheromone biology of Galerucella calmariensis and relationship to dispersal and colonization. Biol. Control 2008, 46, 409–416. [Google Scholar] [CrossRef]
- Weber, D.C.; Konstantinov, A.S.; Khrimian, A.; Bier, A.D.; Lubenow, L.A.; Knodel, J.J.; Haber, A.I.; Wallingford, A.K.; Mason, J.A.C.; Kuhar, T.P. Trapping of crucifer-feeding flea beetles (Phyllotreta spp.) (Coleoptera: Chrysomelidae) with pheromones and plant kairomones. J. Econ. Entomol. 2022, 115, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Soroka, J.J.; Bartelt, R.J.; Zilkowski, B.W.; Cossé, A.A. Responses of flea beetle Phyllotreta cruciferae to synthetic aggregation pheromone components and host plant volatiles in field trials. J. Chem. Ecol. 2005, 31, 1829–1843. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Yin, C.; Liu, X.; Zhang, Q. Attraction of Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) by several plant volatiles and aggregation pheromone. Acta Entomol. Sin. 2010, 53, 734–740. [Google Scholar]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W.; Bean, D.W.; Andress, E.R. Behaviorally active green leaf volatiles for monitoring the leaf beetle, Diorhabda elongata, a biocontrol agent of saltcedar, Tamarix spp. J. Chem. Ecol. 2006, 32, 2695–2708. [Google Scholar] [CrossRef]
- Giblin-Davis, R.M.; Weissling, T.J.; Oehlschlager, A.C.; Gonzalez, L.M. Field response of Rhynchophorus cruentatus (Coleoptera: Curculionidae) to its aggregation pheromone and fermenting plant volatiles. Fla. Entomol. 1994, 77, 164–177. [Google Scholar] [CrossRef]
- Wibe, A.; Borg-Karlson, A.-K.; Cross, J.; Bichão, H.; Fountain, M.; Liblikas, I.; Sigsgaard, L. Combining 1,4-dimethoxybenzene, the major flower volatile of wild strawberry Fragaria vesca, with the aggregation pheromone of the strawberry blossom weevil Anthonomus rubi improves attraction. Crop Prot. 2014, 64, 122–128. [Google Scholar] [CrossRef]
- Hitchner, E.M.; Kuhar, T.P.; Dickens, J.C.; Youngman, R.R.; Schultz, P.B.; Pfeiffer, D.G. Host plant choice experiments of Colorado potato beetle (Coleoptera: Chrysomelidae) in Virginia. J. Econ. Entomol. 2008, 101, 859–865. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Rodrigo-Gómez, S.; Fernández-Carrillo, E.; Corbella-Martorell, C.; Quero, C. Laboratory Evidence of 2-Isobutyl-3-methoxypyrazine as a Male-Released Aggregative Cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae). Insects 2023, 14, 107. https://doi.org/10.3390/insects14020107
López S, Rodrigo-Gómez S, Fernández-Carrillo E, Corbella-Martorell C, Quero C. Laboratory Evidence of 2-Isobutyl-3-methoxypyrazine as a Male-Released Aggregative Cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae). Insects. 2023; 14(2):107. https://doi.org/10.3390/insects14020107
Chicago/Turabian StyleLópez, Sergio, Sara Rodrigo-Gómez, Enrique Fernández-Carrillo, Clàudia Corbella-Martorell, and Carmen Quero. 2023. "Laboratory Evidence of 2-Isobutyl-3-methoxypyrazine as a Male-Released Aggregative Cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae)" Insects 14, no. 2: 107. https://doi.org/10.3390/insects14020107
APA StyleLópez, S., Rodrigo-Gómez, S., Fernández-Carrillo, E., Corbella-Martorell, C., & Quero, C. (2023). Laboratory Evidence of 2-Isobutyl-3-methoxypyrazine as a Male-Released Aggregative Cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae). Insects, 14(2), 107. https://doi.org/10.3390/insects14020107