Medfly Population Suppression through Augmentative Release of an Introduced Parasitoid in an Irrigated Multi-Fruit Orchard of Central–Western Argentina
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Insects and Rearing Procedures
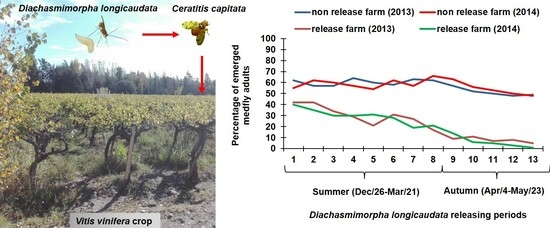
2.2. Experimental Locations and Study Area Description
2.3. Parasitoid Release Procedure
2.4. Fly and Parasitoid Monitoring
2.5. Traps and OD Laboratory Processing
2.6. Weather Conditions during the Study
2.7. Statistical Analyses
2.7.1. Database Management and Statistical Descriptions
2.7.2. GLS Modeling
3. Results
3.1. Statistical Description and Correlations among Tested Variables
3.2. Medfly Performance and Temperature
3.3. Medfly Performance and Temporal Variation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicácio, J.; Abot, A.R.; Oliveira, M.P.; Silva, J.L.; Garcia, F.R.M. Spatial distribution of Ceratitis capitata in guava orchards and influences from orchard management. Braz. J. Biol. 2022, 82, e263741. [Google Scholar] [CrossRef] [PubMed]
- Nicácio, J.; Abot, A.R.; Oliveira, M.P.; Silva, J.L.; Garcia, F.R.M. Sequential sampling plan for Ceratitis capitata (Diptera: Tephritidae) in guava orchards. Anais Acad. Brasil. Ci. 2022, 94, 1–7. [Google Scholar] [CrossRef]
- Cayol, J.P.; Causse, R. Mediterranean fruit fly Ceratitis capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J. Appl. Entomol. 1993, 116, 94–100. [Google Scholar] [CrossRef]
- Enkerlin, W.; Gutiérrez-Ruelas, J.M.; Villaseñor, A.; Cotoc, E.; Midgarden, D.; Lira, E.; Zavala, J.L.; Hendrichs, J.; Liedo, P.; Trujillo, J. Area freedom in México from Mediterranean fruit fly (Diptera: Tephritidae): A review of over 30 years of a successful containment program using an integrated area-wide SIT approach. Fla. Entomol. 2015, 98, 665–681. [Google Scholar] [CrossRef]
- Hallman, G.J.; Quinlan, M. Synopsis of postharvest quarantine research. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1996; pp. 473–477. [Google Scholar]
- CABI Centre for Agricultural Bioscience International. Invasive Species Compendium, Ceratitis capitata (Mediterranean Fruit Fly). CAB International. 2022. Available online: https://www.cabi.org./isc/datasheet/12367 (accessed on 13 January 2023).
- Copeland, R.S.; Wharton, R.A.; Luke, Q.; De Meyer, M. Indigenous hosts of Ceratitis capitata (Diptera: Tephritidae) in Kenya. Ann. Entomol. Soc. Am. 2002, 95, 672–694. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Terblanche, J.S. Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): Effects of age, gender and feeding status. J. Therm. Biol. 2009, 34, 406–414. [Google Scholar] [CrossRef]
- König, S.; Steinmöller, S.; Baufeld, P. Origin and potential for overwintering of Ceratitis capitata (Wiedemann) captured in an official survey in Germany. J. Plant. Dis. Prot. 2022, 129, 1201–1215. [Google Scholar] [CrossRef]
- Diamantidis, A.; Carey, J.R.; Nakas, C.T.; Papadopoulos, N.T. Population-specific demography and invasion potential in medfly. Ecol. Evol. 2011, 1, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Schliserman, P.; Aluja, M.; Rull, J.; Ovruski, S.M. Habitat degradation and introduction of exotic plants favor persistence of invasive species and population growth of native polyphagous fruit fly pests in a Northwestern Argentinean mosaic. Biol. Invasions 2014, 16, 2599–2613. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Schliserman, P.; Aluja, M. Native and introduced host plants of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in Northwestern Argentina. J. Econ. Entomol. 2003, 96, 1108–1118. [Google Scholar] [CrossRef]
- Guillén, D.; Sánchez, R. Expansion of the National Fruit Fly Control Programme in Argentina. In Area-Wide Control of Insects Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. [Google Scholar]
- Bouvet, J.P.; Segade, G. Arándanos y Cítricos: Anastrepha fraterculus (Wiedemann) y Ceratitis capitata (Wiedemann). In Plagas Cuarentenarias de frutales de la República Argentina: Avances en los Resultados; Rossini, M., Agostini, J.P., Dummel, D.M., Eds.; Ediciones del Instituto Nacional de Tecnología Agropecuaria, Centro Regional Patagonia Norte, Estación Experimental Agropecuaria Alto Valle: Río Negro, Argentina, 2015; pp. 31–43. [Google Scholar]
- Funes, C.F.; Escobar, L.I.; Meneguzzi, N.G.; Ovruski, S.M.; Kirschbaum, D.S. Occurrence of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in organically grown Rubus spp. (Rosales: Rosaceae), in two very contrasting environments of northwestern Argentina. Fla. Entomol. 2017, 100, 672–674. [Google Scholar] [CrossRef]
- SENASA; Servicio Nacional de Sanidad y Calidad Agroalimentaria. Moscas de los Frutos. 2017. Available online: http://www.senasa.gob.ar/cadena-vegetal/frutales/produccion-primaria/programas-fitosanitarios/mosca-de-los-frutos-0 (accessed on 1 June 2022).
- Quiroga, D.; Ramirez, W.; Ruiz, C. National Fruit Fly Control and Eradication Program (PROCEM) Argentina. In Proceedings of the 8th International Symposium on Fruit Flies of Economic Importance, Valencia, Spain, 26 September–1 October 2010; Sabater-Muñóz, B., Navarro-Llopis, V., Urbaneja, A., Eds.; Editorial Universitat Politécnica de Valéncia: Valencia, Spain, 2010; p. 100. Available online: http://hdl.handle.net/10251/11200 (accessed on 1 June 2022).
- SENASA; Servicio Nacional de Sanidad y Calidad Agroalimentaria. Control de 1 Millón de Hectáreas para Prevenir la Mosca de los Frutos. 2018. Available online: http://www.senasa.gob.ar/senasa-comunica/infografias/control-de-1-millon-de-hectareas-para-prevenir-la-mosca-de-los-frutos (accessed on 6 June 2022).
- Alós, M.; Llera, H.; Taret, G. Resultados del cambio de estrategia adoptada desde 2011. In Programa de Control y Erradicación de Mosca de los Frutos; Gobierno de San Juan: San Juan, Argentina, 2014; 14p. [Google Scholar]
- Sánchez, G.; Murúa, F.; Suárez, L.; Van Nieuwenhove, G.; Taret, G.; Pantano, V.; Ovruski, S.M. Augmentative releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for Ceratitis capitata (Diptera: Tephritidae) control in a fruit-growing region of Argentina. Biol. Control. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the implications for mass rearing and augmentative release. Insects 2012, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Montoya, P.; López, P.; Cruz, J.; López, F.; Cadena, C.; Cancino, J.; Liedo, P. Effect of Diachasmimorpha longicaudata releases on the native parasitoid guild attacking Anastrepha spp. larvae in disturbed zones of Chiapas, Mexico. BioControl 2017, 62, 581–593. [Google Scholar] [CrossRef]
- Sivinski, J. Augmentative biological control: Research and methods to help make it work. CAB Rev. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- CABI Bioprotection Portal. 5 Advantages of Biocontrol Compared to Chemical Pest Control. Available online: https://bioprotectionportal.com/es/blog/2022/5-advantages-of-biocontrol-over-chemical-control (accessed on 4 March 2023).
- Suárez, L.; Buonocore Biancheri, M.J.; Murúa, F.; Bilbao, M.; García, M.; Cancino, J.; Martín, O.; Molina, D.; Laría, O.; Ovruski, S.M. Effects of host age and radiation dose in Diachasmimorpha longicaudata (Hymenoptera: Braconidae) mass reared on Medfly larvae of the tsl Vienna 8 genetic sexing strain. Biol. Control 2019, 130, 51–59. [Google Scholar] [CrossRef]
- de Pedro, L.; Tormos, J.; Harbi, A.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Beitia, F. Combined use of the larvo-pupal parasitoids Diachasmimorpha longicaudata and Aganaspis daci for biological control of the medfly. Ann. Appl. Biol. 2019, 174, 40–50. [Google Scholar] [CrossRef]
- Vargas, R.I.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional suppression of bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Gómez, E.; Villaseñor, A. Parasitoid releases in the control of Ceratitis capitata (Diptera: Tephritidae) outbreaks, in coffee growing zones of Chiapas, México. Vedalia 2005, 12, 85–89. [Google Scholar]
- Cancino, J.; Ruíz, L.; López, E.; Aguilar, E.; Gálvez, C.; Montoya, P.; Liedo, P. Suppression of Ceratitis capitata (Wied.) (Diptera: Tephritidae) populations in coffee in the Mexico-Guatemala border region through the augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biocontrol Sci. Techn. 2019, 29, 822–826. [Google Scholar] [CrossRef]
- Burns, R.E.; Diaz, J.D.; Holler, T.C. Inundative release of the parasitoid Diachasmimorpha longicaudata for the control of the Caribbean fruit fly, Anastrepha suspensa. In A World Assessment of Their Biology and Management; Mcpheron, B.A., Steck, G.J., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1996; pp. 377–381. [Google Scholar]
- Sivinski, J.; Calkins, C.O.; Baranowski, R.; Harris, D.; Brambila, J.; Diaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of a Caribbean fruit fly (Anastrepha suspensa) (Loew) (Diptera: Tephritidae) population through augmentative releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Sivinski, J.; Aluja, M. Biological Control of Anastrepha spp. (Diptera: Tephritidae) in Mango Orchards Through Augmentative Releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 212–224. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative biological control component in the Mexican national campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. [Google Scholar]
- Garcia, F.R.M.; Ricalde, M.P. Augmentative biological control using parasitoids for fruit fly management in Brazil. Insects 2013, 4, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.; Murúa, F.; Lara, N.; Escobar, J.; Taret, G.; Rubio, J.L.; Van Nieuwenhove, G.; Bezdjian, L.; Schliserman, P.; Ovruski Alderete, S.M. Biological control of Ceratitis capitata (Diptera: Tephritidae) in Argentina: Releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) in fruit-producing semi-arid areas of San Juan. Nat. Sci. 2014, 6, 664–675. [Google Scholar]
- Wong, T.T.Y.; Ramadan, M.M.; McInnis, D.O.; Mochizuki, N.; Nishimoto, J.J.; Herr, J.C. Augmentative releases of Diachasmimorpha tryoni (Hymenoptera: Braconidae) to suppress a Mediterranean fruit fly (Diptera: Tephritidae) population in Kula, Maui, Hawaii. Biol. Control 1991, 1, 2–7. [Google Scholar] [CrossRef]
- Sivinski, J.; Jeronimo, F.; Holler, T. Development of aerial releases of Diachasmimorpha tryoni (Cameron) (Hymenoptera: Braconidae), a Parasitoid that attacks the Mediterranean Fruit Fly, Ceratitis capitata (Weidemann) (Diptera: Tephritidae), in the Guatemalan Highlands. Biocontrol Sci. Technol. 2000, 10, 15–25. [Google Scholar] [CrossRef]
- Vargas, R.I.; Peck, S.L.; McQuate, G.T.; Jackson, C.G.; Stark, J.D.; Armstrong, J.W. Potential for area-wide integrated management of mediterranean fruit fly (Diptera: Tephritidae) with a braconid parasitoid and a novel bait spray. J. Econ. Entomol. 2001, 94, 817–825. [Google Scholar] [CrossRef]
- Wang, X.G.; Messing, R.H. Potential interactions between pupal and egg- or larval-pupal parasitoids of tephritid fruit flies. Environ. Entomol. 2004, 33, 1313–1320. [Google Scholar] [CrossRef]
- Rendon, P.; Sivinski, J.; Holler, T.; Bloem, K.; López, M.; Matinez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha kraussii (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (Wied.) (Diptera: Tephritidae) at various sites in Guatemala. Biol. Control 2006, 36, 224–231. [Google Scholar]
- Cáceres, C. Mass rearing of temperate sensitive genetic sexing strains in the Mediterranean Fruit Fly (Ceratitis capitata). Genetica 2002, 116, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, J.S.; Nyamukondiwa, C.; Kleynhans, E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomol. Exp. Appl. 2010, 137, 304–315. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; p. 528. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; p. 504. [Google Scholar]
- Galecki, A.T.; Burzykowski, T. Linear Mixed-Effects Models Using R. In A Step-by-Step Approach; Springer: New York, NY, USA, 2013; p. 542. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Pinheiro, J.; Bates, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3. 2022, p. 158. Available online: https://CRAN.R-project.org/package=nlme (accessed on 10 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; p. 2630. Available online: https://www.R-project.org/ (accessed on 10 May 2022).
- Suárez, L.; Biancheri, M.J.; Díaz Nieto, L.M.; Schliserman, P.; Murúa, F.; Rull, J.; Lasa, R.; Pantano, V.; Ovruski, S.M. Dynamic seasonal response of Ceratitis capitata (Diptera: Tephritidae) to fruit juice-based lures in fig orchards. Int. J. Pest Manag. 2021. [Google Scholar] [CrossRef]
- Chinajariyawong, A.; Clarke, A.R.; Jirasurat, M.; Kritsaneepiboon, S.; Lahey, H.A.; Vijaysegaran, S.; Walter, G.H. Survey of opiine parasitoids of fruit flies in Thailand and Malaysia. Raffles Bull. Zool. 2000, 48, 71–101. [Google Scholar]
- Ovruski, S.M.; Aluja, M.; Sivinski, J. Hymenopteran parasitoids on fruit infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, distribution, taxonomic status and their use in fruit fly biological control. Integr. Pest Manag. Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]




| Variables | Farm Conditions/Study Seasons | |||
|---|---|---|---|---|
| Non-Parasitoid Release | Parasitoid Release | |||
| 2013 | 2014 | 2013 | 2014 | |
| No. of recovered medfly puparia | 20.7 ± 5.9 | 21.9 ± 3.8 | 15.7 ± 7.2 | 12.8 ± 5.2 |
| No. of emerged medfly adults | 12.0 ± 4.2 | 12.7 ± 2.9 | 4.4 ± 3.4 | 3.2 ± 2.6 |
| No. of emerged parasitoid adults | 0 | 0 | 2.7 ± 1.3 | 3.5 ± 1.2 |
| No. of non-emerged medfly puparia | 8.7 ± 1.8 | 9.2 ± 1.3 | 8.6 ± 3.4 | 6.0 ± 1.6 |
| Medfly emergence (%) | 56.7 ± 5.2 | 57.2 ± 5.0 | 22.0 ± 12.9 | 20.4 ± 12.7 |
| Parasitism (%) | 0 | 0 | 18.3 ± 4.7 | 27.1 ± 4.7 |
| Medfly pupal mortality (%) | 43.1 ± 12.0 | 41.9 ± 11.4 | 76.2 ± 19.4 | 75.2 ± 22.0 |
| FTD index | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.7 ± 0.3 | 0.6 ± 0.3 |
| No. of released female parasitoids | 0 | 0 | 8861.5 ± 312.1 | 8826.9 ± 251.3 |
| Air temperature (°C) | 21.9 ± 4.6 | 22.7 ± 6.2 | 21.8 ± 4.9 | 22.7 ± 6.2 |
| Air relative humidity (%) | 54.6 ± 7.8 | 56.2 ± 7.4 | 54.1 ± 7.4 | 56.1 ± 7.2 |
| Accumulative rainfall (mm) | 7.9 ± 12.3 | 11.5 ± 12.8 | 8.0 ± 12.5 | 11.4 ± 12.6 |
| First Response Variable | Second Response Variables | Person’s Correlation Results | ||||
|---|---|---|---|---|---|---|
| r | Lower Confidence Limit | Upper Confidence Limit | t | p | ||
| Medfly emergence | No. of recovered medfly puparia | 0.740 | 0.489 | 0.874 | 5.337 | <0.001 |
| No. of emerged medfly adults | 0.850 | 0.695 | 0.932 | 8.002 | <0.001 | |
| No. of non-emerged medfly puparia | 0.360 | −0.026 | 0.659 | 1.919 | =0.067 | |
| FTD | 0.620 | 0.307 | 0.813 | 3.880 | <0.001 | |
| Air temperature | 0.620 | 0.301 | 0.810 | 3.836 | <0.001 | |
| Air relative humidity | −0.460 | −0.721 | −0.091 | −2.553 | =0.017 | |
| Cumulative rainfall | 0.170 | −0.231 | 0.524 | 0.855 | =0.410 | |
| Parasitism | No. of released female parasitoids | 0.210 | −0.195 | 0.551 | 1.042 | =0.308 |
| Air temperature | −0.089 | −0.460 | 0.309 | −0.436 | =0.667 | |
| Air relative humidity | 0.290 | −0.106 | 0.611 | 1.505 | =0.145 | |
| Cumulative rainfall | −0.110 | −0.481 | 0.285 | −0.566 | =0.576 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, L.; Biancheri, M.J.B.; Murúa, F.; Ordano, M.; Wang, X.; Cancino, J.; Garcia, F.R.M.; Sánchez, G.; Beltrachini, S.; Kulichevsky, L.E.; et al. Medfly Population Suppression through Augmentative Release of an Introduced Parasitoid in an Irrigated Multi-Fruit Orchard of Central–Western Argentina. Insects 2023, 14, 387. https://doi.org/10.3390/insects14040387
Suárez L, Biancheri MJB, Murúa F, Ordano M, Wang X, Cancino J, Garcia FRM, Sánchez G, Beltrachini S, Kulichevsky LE, et al. Medfly Population Suppression through Augmentative Release of an Introduced Parasitoid in an Irrigated Multi-Fruit Orchard of Central–Western Argentina. Insects. 2023; 14(4):387. https://doi.org/10.3390/insects14040387
Chicago/Turabian StyleSuárez, Lorena, María Josefina Buonocore Biancheri, Fernando Murúa, Mariano Ordano, Xingeng Wang, Jorge Cancino, Flavio Roberto Mello Garcia, Guillermo Sánchez, Sergio Beltrachini, Luis Ernesto Kulichevsky, and et al. 2023. "Medfly Population Suppression through Augmentative Release of an Introduced Parasitoid in an Irrigated Multi-Fruit Orchard of Central–Western Argentina" Insects 14, no. 4: 387. https://doi.org/10.3390/insects14040387
APA StyleSuárez, L., Biancheri, M. J. B., Murúa, F., Ordano, M., Wang, X., Cancino, J., Garcia, F. R. M., Sánchez, G., Beltrachini, S., Kulichevsky, L. E., & Ovruski, S. M. (2023). Medfly Population Suppression through Augmentative Release of an Introduced Parasitoid in an Irrigated Multi-Fruit Orchard of Central–Western Argentina. Insects, 14(4), 387. https://doi.org/10.3390/insects14040387