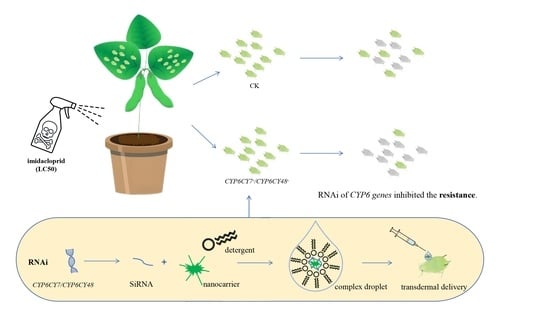
RNA Interference Reveals the Impacts of CYP6CY7 on Imidacloprid Resistance in Aphis glycines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture and Insecticide Used
2.2. CYP6 Sequence Cloning and Analysis
2.3. CYP6 Expression Pattern Due to Imidacloprid Treatment Induction
2.4. Study of the Function of A. glycines CYP6s during Imidacloprid Treatment
2.5. Statistical Analysis
3. Results
3.1. Identification of Twelve CYP6 Sequences
3.2. Differential Expression of CYP6s in Response to Imidacloprid Treatment Induction
3.3. Relative Expressions of CYP6CY7 after RNAi
3.4. P450 Enzyme Activities after RNA Inhibition
3.5. Sensitivity of A. glycines to Imidacloprid Treatment after RNA Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilmon, K.J.; Hodgson, E.W.; O’Neal, M.E.; Ragsdale, D.W. Biology of the soybean aphid, Aphis glycines (Hemiptera: Aphididae) in the United States. J. Integr. Agric. 2011, 2, A1–A7. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, D.W.; McCornack, B.P.; Venette, R.C.; Potter, B.D.; MacRae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’Neil, R.J.; DiFonzo, C.D.; et al. Economic threshold for soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.; Foottit, R.; Gagnier, D.; Baute, T. First Canadian records of Aphis glycines (Hemiptera: Aphididae). Can. Entomol. 2003, 135, 879–881. [Google Scholar] [CrossRef]
- Hill, J.H.; Alleman, R.; Hogg, D.B.; Grau, C.R. First Report of Transmission of Soybean mosaic virus and Alfalfa mosaic virus by Aphis glycines in the New World. Plant Dis. 2001, 85, 561. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Perry, K.L. Transmissibility of Field Isolates of Soybean Viruses by Aphis glycines. Plant Dis. 2002, 86, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Venette, R.C.; Ragsdale, D.W. Assessing the Invasion by Soybean Aphid (Homoptera: Aphididae): Where Will It End? Ann. Entomol. Soc. Am. 2004, 97, 219–226. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, K.J. Biology and control techniques of soybean aphid, Aphis glycines. Chin. Bull. Entomol. 2007, 44, 179–185. [Google Scholar]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef]
- Tang, J.J.; Chen, X.; Zhang, C.J.; Wang, X. Advances of Imidacloprid Research and Its Applicationin in Crop Pest Control. Chin. Agr. Sci. Bull. 1999, 15, 38–40. [Google Scholar]
- Le Questel, J.Y.; Graton, J.; Cerón-Carrasco, J.P.; Jacquemin, D.; Planchat, A.; Thany, S.H. New insights on the molecular features and electrophysiological properties of dinotefuran, imidacloprid and acetamiprid neonicotinoid insecticides. Bioorg. Med. Chem. 2011, 19, 7623–7634. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Tang, M.; Wang, J.J.; Du, B.; Lu, D.M.; Wang, Z.Y. Research Summary in Neonicotinoids Insecticides. Shandong Chem. Ind. 2015, 44, 66–68. [Google Scholar]
- Zhang, A.N.; Han, L.L.; Zhao, K.J.; Zhang, W.L.; Xiao, J.F.; Chen, J.; Gao, L.T. Effects of semilethal and sublethal doses of imidacloprid on Aphis glycines (Hemiptera: Aphididae). Chin. J. Appl. Entomol. 2020, 57, 676–681. [Google Scholar]
- Xu, L.; Wang, J.H.; Mei, Y.; Li, D.Z. Research progress on the molecular mechanisms of insecticides resistance mediated by detoxification enzymes and transporters. Chin. J. Pestic. Sci. 2020, 22, 1–10. [Google Scholar]
- Chen, M.H.; Han, Z.J.; Qiao, X.F.; Qu, M.J. Mutations in acetylcholinesterase genes of Rhopalosiphum padi resistant to organophosphate and carbamate insecticides. Genome 2007, 50, 172–179. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X. Multiple mechanisms responsible for differential susceptibilities of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus) to pirimicarb. Bull. Entomol. Res. 2009, 99, 611–617. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest. Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Kang, X.L.; LI, Y.T.; Wang, K.; Zhang, M.; Duan, X.L.; Peng, X.; Chen, M.H. Molecular cloning, characterization and expression analysis of ATPbinding cassette transporter genes in the bird cherry-oat aphid, Rhopalosiphum padi(Hemiptera: Aphididae). Acta Entomol. Sin. 2015, 58, 593–602. [Google Scholar]
- Yu, S.J. Detoxification Mechanisms in Insects. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Omura, T.; Ryo Sato, R. The Carbon Monoxide-binding Pigment of Liver Microsomes: I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Scott, J.G.; Liu, N.; Wen, Z. Insect cytochromes P450: Diversity, insecticide resistance and tolerance to plant toxins. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 121, 147–155. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef]
- Karunker, I.; Morou, E.; Nikou, D.; Nauen, R.; Sertchook, R.; Stevenson, B.J.; Paine, M.J.; Morin, S.; Vontas, J. Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem. Mol. Biol. 2009, 39, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Gao, H.; Zhang, Y.; Fan, D.; Fang, J.; Liu, Z. The roles of CYP6AY1 and CYP6ER1 in imidacloprid resistance in the brown planthopper: Expression levels and detoxification efficiency. Pestic. Biochem. Physiol. 2016, 129, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fan, X.; Gao, Y.; Yang, J.; Qian, J.; Fan, D. Identification of two novel P450 genes and their responses to deltamethrin in the cabbage moth, Mamestra brassicae Linnaeus. Pestic. Biochem. Physiol. 2017, 141, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Zhu, W.H.; Liao, W.L.; BU, X.L.; Jiang, W.Y. Ds rna delivery methods for rna interference in insects. Acta Entomol. Sin. 2016, 59, 685–691. [Google Scholar]
- He, B.; Chu, Y.; Yin, M.; Müllen, K.; An, C.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bansal, R.; Mamidala, P.; Mian, M.A.; Mittapalli, O.; Michel, A.P. Validation of reference genes for gene expression studies in Aphis glycines (Hemiptera: Aphididae). J. Econ. Entomol. 2012, 105, 1432–1438. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest. Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; E-Publishing Inc.: London, UK, 2012; pp. 236–316. [Google Scholar]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Naseri, B.; Abedi, Z.; Setzer, W.N.; Changbunjong, T. Promising Insecticidal Efficiency of Essential Oils Isolated from Four Cultivated Eucalyptus Species in Iran against the Lesser Grain Borer, Rhyzopertha dominica (F.). Insects 2022, 13, 517. [Google Scholar] [CrossRef]
- Khan, S.; Uddin, M.N.; Rizwan, M.; Khan, W.; Farooq, M.; Shah, A.S.; Muhammad, M. Mechanism of insecticide resistance in insects/pests. Pol. J. Environ. Stud. 2020, 29, 2023–2030. [Google Scholar] [CrossRef]
- Strode, C.; Wondji, C.S.; David, J.P.; Hawkes, N.J.; Lumjuan, N.; Nelson, D.R.; Drane, D.R.; Karunaratne, S.H.; Hemingway, J.; Black, W.C., 4th; et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2008, 38, 113–123. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, X.; Sun, Y.; Ma, L.; Shen, B.; Zhu, C. Genomic Analysis of Detoxification Supergene Families in the Mosquito Anopheles sinensis. PLoS ONE 2015, 10, e0143387. [Google Scholar] [CrossRef]
- Pittendrigh, B.; Aronstein, K.; Zinkovsky, E.; Andreev, O.; Campbell, B.; Daly, J.; Trowell, S.; Ffrench-Constant, R.H. Cytochrome P450 genes from Helicoverpa armigera: Expression in a pyrethroid-susceptible and -resistant strain. Insect Biochem. Mol. Biol. 1997, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Dong, H.Q.; Tian, H.S.; Ma, L.; Li, X.L.; Wu, G.L.; Zhu, C.L. Cytochrome P450 genes expressed in the deltamethrin-susceptible and resistant strains of Culex pipiens pallens. Pestic. Biochem. Physiol. 2003, 75, 19–26. [Google Scholar] [CrossRef]
- Zeng, X.; Pan, Y.; Song, J.; Li, J.; Lv, Y.; Gao, X.; Tian, F.; Peng, T.; Xu, H.; Shang, Q. Resistance Risk Assessment of the Ryanoid Anthranilic Diamide Insecticide Cyantraniliprole in Aphis gossypii Glover. J. Agric. Food Chem. 2021, 69, 5849–5857. [Google Scholar] [CrossRef]
- Lv, Y.; Wen, S.; Ding, Y.; Gao, X.; Chen, X.; Yan, K.; Yang, F.; Pan, Y.; Shang, Q. Functional Validation of the Roles of Cytochrome P450s in Tolerance to Thiamethoxam and Imidacloprid in a Field Population of Aphis gossypii. J. Agric. Food Chem. 2022, 16, 14339–14351. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.H.; Mao, K.K.; Liao, X.; Xu, P.F.; Li, Z.; Ali, E.; Wan, H. Overexpression of CYP6ER1 associated with clothianidin resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 154, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.A.; Miyata, T.; Miura, K.; Tanaka, T. RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect Biochem. Mol. Biol. 2009, 39, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest. Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Mizoguchi, T.; Fujiwara, H. siRNAs induce efficient RNAi response in Bombyx mori embryos. PLoS ONE 2011, 6, e25469. [Google Scholar] [CrossRef]
- Lares, M.R.; Rossi, J.J.; Ouellet, D.L. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010, 28, 570–579. [Google Scholar] [CrossRef]
- Gong, L.; Yang, X.; Zhang, B.; Zhong, G.; Hu, M. Silencing of Rieske iron-sulfur protein using chemically synthesised siRNA as a potential biopesticide against Plutella xylostella. Pest. Manag. Sci. 2011, 67, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, G.P.; Rajam, M.V. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 2009, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Clemons, A.; Haugen, M.; Le, C.; Mori, A.; Tomchaney, M.; Severson, D.W.; Duman-Scheel, M. siRNA-mediated gene targeting in Aedes aegypti embryos reveals that frazzled regulates vector mosquito CNS development. PLoS ONE 2011, 6, e16730. [Google Scholar] [CrossRef] [PubMed]





| Gene Name | Accession No. | Length (bp) | Length (aa) | pI | Mw (kDa) |
|---|---|---|---|---|---|
| CYP6CY7 | MN055997 | 1735 | 517 | 8.40 | 59.20 |
| CYP6CY8 | MN056000 | 1913 | 514 | 8.60 | 59.50 |
| CYP6CY9 | MN055999 | 2071 | 522 | 8.34 | 59.44 |
| CYP6CY12 | MN055998 | 1686 | 505 | 8.68 | 58.21 |
| CYP6CY14 | MN056003 | 2468 | 513 | 7.56 | 58.96 |
| CYP6CY16 | MN055995 | 1769 | 513 | 8.38 | 59.27 |
| CYP6CY18 | MN056002 | 1556 | 508 | 8.76 | 59.38 |
| CYP6CY20 | MG829856 | 2040 | 509 | 9.10 | 59.20 |
| CYP6CY48 | MN055996 | 1639 | 511 | 8.87 | 59.48 |
| CYP6CZ1 | MN055993 | 1837 | 511 | 8.86 | 59.37 |
| CYP6DB1 | MN056001 | 2287 | 513 | 9.11 | 59.58 |
| CYP6DD1 | MN055994 | 2108 | 512 | 8.27 | 58.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, H.; Wang, Y.; Wei, L.; Lyu, J.; Shan, Z.; Zhang, X.; Fan, D. RNA Interference Reveals the Impacts of CYP6CY7 on Imidacloprid Resistance in Aphis glycines. Insects 2024, 15, 188. https://doi.org/10.3390/insects15030188
Li S, Yang H, Wang Y, Wei L, Lyu J, Shan Z, Zhang X, Fan D. RNA Interference Reveals the Impacts of CYP6CY7 on Imidacloprid Resistance in Aphis glycines. Insects. 2024; 15(3):188. https://doi.org/10.3390/insects15030188
Chicago/Turabian StyleLi, Shuangyu, Hongjia Yang, Yixiao Wang, Lisi Wei, Jiawei Lyu, Zhimeng Shan, Xinxin Zhang, and Dong Fan. 2024. "RNA Interference Reveals the Impacts of CYP6CY7 on Imidacloprid Resistance in Aphis glycines" Insects 15, no. 3: 188. https://doi.org/10.3390/insects15030188
APA StyleLi, S., Yang, H., Wang, Y., Wei, L., Lyu, J., Shan, Z., Zhang, X., & Fan, D. (2024). RNA Interference Reveals the Impacts of CYP6CY7 on Imidacloprid Resistance in Aphis glycines. Insects, 15(3), 188. https://doi.org/10.3390/insects15030188