Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization
2.3. Electrochemical Techniques
3. Results
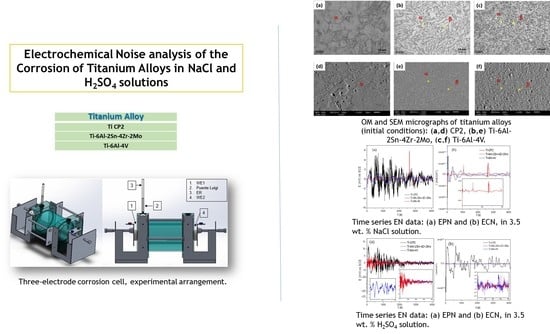
3.1. OM-SEM Microstructural Analysis
3.2. Electrochemical Noise
3.2.1. Statistical Analysis
3.2.2. PSD Analysis
3.2.3. Wavelet Method
3.2.4. HHT Analysis
3.2.5. SEM Corrosion Product Analysis
4. Discussion
5. Conclusions
- In this work, characterization by electrochemical noise of titanium alloys could find potential applications in the aeronautical industry. Ti-Alloys developed a passive layer in both electrolytes, but this one is more stable in H2SO4 than in NaCl solution.
- EN results show that Rn has higher values for Ti-6Al-2Sn-4Zr-2Mo and Ti-6Al-4V in H2SO4 than in NaCl solutions. The current and potential time series show different behavior for each electrolyte, and anodic–cathodic transients’ fluctuations can be associated with a general or localized corrosion process.
- Skewness and kurtosis results show that they must be interpreted as a measure of the disorder and distribution of transients and not as a mechanistic method for Ti alloys. This also generated discrepancies due to the limitations of statistical analysis for chaotic systems.
- PSD in current results showed that Ti-6Al-2Sn-4Zr-2Mo has more resistance to dissolution alloy because it presents lower values of Ψ0. Limit frequency all Ti-alloys presented a changed passivation process.
- Wavelets and HHT methods are more reliable to determine the type of corrosion for Ti alloys than statistical methods. In NaCl and H2SO4, wavelets and HHT presented similar results. For NaCl, behavior was associated with a slow process, but the energy presence at middle frequencies was significant and an unstable passive layer was attributed to Cl− ions. Alloys manifested in H2SO4 a slow process associated with passivation in wavelets and HHT methods.
- SEM-EDS observations indicated that wavelets and HHT in Ti-alloys exposed in NaCl solution showed the presence of Cl− as agent diffusion, and when alloys were exposed to H2SO4 solution, the surface morphology had the presence of oxygen, a main element of the passivation layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Froes, F.H. Titanium Physical Metallurgy Processing and Applications, 1st ed.; Froes, F.H., Ed.; ASM International, Materials Park: Russell, OH, USA, 2015; pp. 75–84. [Google Scholar]
- Sha, W.W.; Malinov, S. Titanium Alloys: Modelling of Microstructure, Properties and Applications, 1st ed.; Malinov, S., Ed.; CRC Press: Oxford, UK, 2009; pp. 237–255. [Google Scholar]
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 129–189. [Google Scholar]
- Mouritz, P.A. Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012; pp. 202–223. [Google Scholar]
- Donachie, M.J., Jr. Titanium a Technical Guide, 2nd ed.; Dragolish, K., Ed.; ASM International, Materials Park: Russell, OH, USA, 2000; pp. 123–127. [Google Scholar]
- Barington, N.; Black, M. Aerospace Materials and Manufacturing Processes at the Millennium. In Aerospace Materials; Cantor, B., Assender, H., Grant, P., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 3–15. [Google Scholar]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Semian, S.; Seetharaman, V.; Weiss, I. The thermomechanical processing of alpha/beta titanium alloys. JOM 1997, 49, 33–39. [Google Scholar] [CrossRef]
- Koshal, D. Metal Casting and Moulding Processes. In Manufacturing Engineer’s Reference Book; Koshal, D., Ed.; Butterworth Heinemann: Brighton, UK, 1993; pp. 1–23. [Google Scholar]
- Yang, X.; Liu, C.R. Machining titanium and its alloys. Mach. Sci. Technol. 1997, 3, 107–139. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; Ahmad, Z., Ed.; Butterworth-Heinemann: London, UK, 2006; pp. 57–199. [Google Scholar]
- Noel, J.J.; Shoesmith, D.W.; Ebrahimi, N. Corrosion of Titanium, and Its Alloys, 1st ed.; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 193–199. [Google Scholar]
- Moiseyev, V.N. Titanium Alloys Russian Aircraft and Aerospace Applications, 1st ed.; Fridlayander, J.N., Eskin, D.G., Eds.; Taylor & Francis; CRC Press: Boca Raton, FL, USA, 2005; pp. 47–84. [Google Scholar]
- Noël, J.J. The Electrochemistry of Titanium Corrosion. Ph.D. Thesis, The University of Manitoba, Winnipeg, MB, Canada, 1999. [Google Scholar]
- Tang, X.; Ahmed, T.; Rack, H.J. Phase transformations in Ti-Nb-Ta and Ti-Nb-Ta-Zr alloys. J. Mater. Sci. 2000, 35, 1805–1811. [Google Scholar] [CrossRef]
- Schutz, R.W. Corrosion of Titanium and Titanium Alloys. In ASM Handbook Volume 13B Corrosion: Materials; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International, Materials Park: Russell, OH, USA, 2005; pp. 252–290. [Google Scholar]
- Fu, T.; Zhan, Z.; Tang, Y.; Liu, Z.; Liu, Z.; Li, L.; Yu, X. Effect of surface mechanical attrition treatment on corrosion resistance of commercial pure titanium. Surf. Coat. Technol. 2015, 280, 129–135. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Kumari, S.; Mohan, M.K.; Pant, B.; Rao, M.N. Degradation behavior of metastable β Ti-15-3 alloy for fastener applications. J. Alloys Comp. 2019, 775, 518–523. [Google Scholar] [CrossRef]
- Ossowska, A.; Sobieszcyk, S.; Supernak, M.; Zielinski, A. Morphology, and properties of nanotubular oxide layer on the “Ti-13Zr-13Nb” alloy. Surf. Coat. Technol. 2014, 258, 1239–1248. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization. I. A theoretical analysis of the shape of the polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A. Keimbildung in nbersättigten Gebilden. Z. Phys. Chem. 1959, 119, 277–3013. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria, II. The kinetic interpretation of the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in heterogeneous equilibria, I. Conditions at the boundary surface of crystalline solids and liquids, and the application of statistical mechanics. Trans. Faraday Soc. 1924, 19, 659–665. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Estupiñán, F.; Zambrano, R.P.; Martínez-Villafañe, A.; Borunda, T.A.; Colás, O.R.; Gaona-Tiburcio, C. Análisis de los transitorios de ruido electroquímico para aceros inoxidables 316 y -DUPLEX 2205 en NaCl y FeCl. Rev. Metal. 2012, 4, 147–156. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coat. 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, G. Electrochemical Techniques in Corrosion Science and Engineering; Taylor & Francis: Boca Raton, FL, USA, 2002; pp. 54–123. [Google Scholar]
- Kearns, J.R.; Eden, D.A.; Yaffe, M.R.; Fahey, J.V.; Reichert, D.L.; Silverman, D.C. ASTM Standardization of Electrochemical Noise Measurement. In Electrochemical Noise Measurement for Corrosion Applications; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 446–471. [Google Scholar]
- Botana, P.J.; Bárcena, M.M.; Villero, Á.A. Ruido Electroquímico: Métodos de Análisis; Septem Ediciones: Cadiz, Spain, 2002; pp. 50–70. [Google Scholar]
- Gaona-Tiburcio, C.; Aguilar, L.M.R.; Zambrano-Robledo, P.; Estupiñán-López, F.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-González, E.; Almeraya-Calderón, F. Electrochemical Noise Analysis of Nickel Based Superalloys in Acid Solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of Corrosion Inhibitors by Electrochemical Noise Analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Park, C.J.; Kwon, H.S. Electrochemical noise analysis of localized corrosion of duplex stainless steel aged at 475 °C. Mater. Chem. Phys. 2005, 91, 355–360. [Google Scholar] [CrossRef]
- Suresh, G.U.; Kamachi, M.S. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion Behavior of AISI 409Nb Stainless Steel Manufactured by Powder Metallurgy Exposed in H2SO4 and NaCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Nagiub, A.M. Electrochemical Noise Analysis for Different Green Corrosion Inhibitors for Copper Exposed to Chloride Media. Port. Electrochim. Acta 2017, 35, 201–210. [Google Scholar] [CrossRef]
- Nakasa, K.; Satoh, H. The effect of hydrogen-charging on the fatigue crack propagation behavior of β-titanium alloys. Corros. Sci. 1996, 38, 457–468. [Google Scholar] [CrossRef]
- Dawson, D.L. Electrochemical Noise Measurement: The definitive In-Situ Technique for Corrosion Applications? In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 3–39. [Google Scholar]
- Cottis, R.; Turgoose, S.; Mendoza-Flores, J. The Effects of Solution Resistance on Electrochemical Noise Resistance Measurements: A Theorical Analysis. In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: Russell, OH, USA, 1996; pp. 93–100. [Google Scholar]
- Brockwell, P.J.; Davis, R.A. Introduction to Time Series and Forecasting, 3rd ed.; Springer: Zürich, Switzerland, 2002; pp. 1–68. [Google Scholar]
- Homborg, A.M.; Tinga, T.; Zhang, X.; Van Westing, E.P.M.; Ferrari, G.M.; Wit, J.H.W.; Mol, J.M.W. A Critical Appraisal of the Interpretation of Electrochemical Noise for Corrosion Studies. Corrosion 2017, 70, 971–987. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti-Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef]
- Du, X.; Yang, Q.S.; Chen, Y.; Zhang, X.Z. Galvanic Corrosion behavior of copper/titanium galvanic couple in artificial seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Vacandio, F.; Fraoucene, H.; Sugiawati, V.A.; Eyraud, M.; Hatem, D.; Belkaid, M.S.; Pasquinelli, M.; Djenizian, T. Optical and Electrochemical Properties of Self-Organized TiO2 Nanotube Arrays from Ti-6Al-4V Alloy. Front. Chem. 2019, 7, 66–83. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, Z.; Liu, C.; Yu, Y. Wear and Electrochemical Corrosion Behavior of Biomedical Ti-5Nb-3Mo-3Zr-2Sn Alloy in Simulated Physiological Solutions. J. Bio. Tribo. Corros. 2015, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yuan, T.; Tang, J.; He, J.; Li, R. Mechanical and corrosion behavior of titanium alloys additively manufactured by selective laser melting—A comparison between nearly β titanium, α titanium and α+β titanium. Opt. Laser Technol. 2019, 119, 105625. [Google Scholar] [CrossRef]
- Dubent, S.; Mazard, A. Characterization and corrosion behaviour of grade 2 titanium used in electrolyzers for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 15622–15633. [Google Scholar] [CrossRef]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- ASTM E407-07. Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Estupiñán-López, H.F.; Almeraya-Calderón, F.; Bautista Margulis, G.R.; Baltazar Zamora, M.A.; Martínez-Villafañe, A.; Uruchurtu, C.J.; Gaona-Tiburcio, C. Transient Analysis of Electrochemical Noise for 316 and Duplex 2205 Stainless Steels Under Pitting Corrosion. Int. J. Electrochem. Sci. 2011, 6, 1785–1796. [Google Scholar]
- Liu, X.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. In-situ study of the formation process of stannate conversion coatings on AZ91D magnesium alloy using electrochemical noise. Corros. Sci. 2010, 52, 892–900. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Basharnavaz, H.; Bezaatpour, A. A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media. Mater. Chem. Phys. 2013, 138, 794–802. [Google Scholar] [CrossRef]
- Eden, D.A.; John, D.G.; Dawson, J.L. Corrosion Monitoring. WO87/07022, 19 November 1987. [Google Scholar]
- Mansfeld, F.; Sun, Z. Technical Note: Localization Index Obtained from Electrochemical Noise Analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Reid, S.A.; Eden, D.A. Assessment of Corrosion. US9264824B1, 24 July 2001. [Google Scholar]
- Cottis, R. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Eden, D.A. Electrochemical Noise—The First Two Octaves. In NACE International Corrosion/98; NACE International: San Diego, FL, USA, 1998; pp. 1–31. [Google Scholar]
- Coakley, J.; Vorontsov, V.A.; Littlell, K.C.; Heenan, R.K.; Ohnuma, G.; Jones, N.G.; Dye, D. Nanoprecipitation in a beta-titanium alloy. J. Alloy. Compd. 2015, 623, 146. [Google Scholar] [CrossRef] [Green Version]
- Bertocci, U.; Huet, F. Noise Analysis Applied to Electrochemical Systems. Corrosion 1995, 51, 131–144. [Google Scholar] [CrossRef]
- Lee, C.C.; Mansfeld, F. Analysis of electrochemical noise data for a passive system in the frequency domain. Corr. Sci. 1998, 40, 959–962. [Google Scholar] [CrossRef]
- Legat, A.; Dolecek, V. Corrosion Monitoring System Based on Measurement and Analysis of electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Uruchurtu, J.C.; Dawson, J.L. Noise Analysis of Pure Aluminum under Different Pitting Conditions. Corrosion 1987, 43, 19–26. [Google Scholar] [CrossRef]
- Lentka, L.; Smulko, J. Methods of trend removal in electrochemical noise data-overview. Measurement 2019, 131, 569–581. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Cao, F.; Gao, Z.; Zhang, J.; Cao, C. Analysis of pitting corrosion behavior of pure Al in sodium chloride solution with the wavelet technique. J. Electroanal. Chem. 2006, 578, 143–150. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.H.; Hu, R.G.; Du, R.G.; Lin, C.J. Study on the corrosion behavior of reinforcing steel in cement mortar by electrochemical noise measurements. Electrochim. Acta 2007, 52, 3976–3984. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, Z.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Lafront, A.M.; Safizadeha, F.; Ghali, E.; Houlachi, G. Study of the cupper anode passivation by electrochemical noise analysis using spectral and wavelet transform. Electrochim. Acta. 2010, 55, 2505–2512. [Google Scholar] [CrossRef]
- Froes, F.; Quian, M.; Niinomi, M. Titanium for Consumer Applications. Real World Use of Titanium; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 27–65. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Adamek, G.; Pałka, K.; Jakubowicz, J. Corrosion properties of Ti scaffolds prepared with sucrose as a space holder. Solid State Phenom. 2015, 227, 519–522. [Google Scholar] [CrossRef]
- Dabrowski, B.; Kaminski, J.; Swieszkowski, W.; Kurzydlowski, K.J. Porous titanium scaffolds for biomedical applications: Corrosion resistance and structure investigation. Mater. Sci. Forum 2011, 674, 41–46. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In vitro studying corrosion behavior of porous titanium coating in dynamic electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef]
- Lohrberg, K.; Kohl, P. Preparation and use of Raney-Ni activated cathodes for large scale hydrogen production. Electrochem. Acta 1984, 29, 1557–1561. [Google Scholar] [CrossRef]
- Huet, F.; Musiani, M.; Nogueira, P. Oxygen evolution on electrodes of different roughness an electrochemical noise study. J. Solid State Electrochem. 2004, 786–793. [Google Scholar] [CrossRef]
- Hai, L.; Guo-Quiang, X.; Pan, Z.; Hua-Sen, Z.; Khan, M.Y. The Hilbert-Huang Transform-Based Denoising Method for the TEM Response of a PRBS Source Signal. Pure App. Geophys. 2016, 173, 2777–2789. [Google Scholar] [CrossRef]
- Homborg, A.M.; Oonincx, P.J.; Mol, J.M.C. Wavelet Transform Modulos Maxima and Holder Exponents Combined with Transient Detection for the Differentiation of Pitting Corrosion Using Electrochemical Noise. Corrosion 2018, 48, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Runa, M.J.; Mathew, M.T.; Rocha, L.A. Tribocorrosion response of the Ti6Al4V alloys commonly used in femoral stems. Tribol. Int. 2013, 68, 85–93. [Google Scholar] [CrossRef]
- Toptan, F.; Rego, A.; Alvces, A.C.; Guedes, A. Corrosion and tribocorrosion behavior of Ti-B4C composite intended for orthopaedic implants. J. Mech. Behav. Biomed. 2016, 61, 152–163. [Google Scholar] [CrossRef]
- Joshi, V.A. Titanium Alloys: An Atlas of Structures and Fracture Features; Joshi, V.A., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 97–140. [Google Scholar]
- Benavides, S. Corrosion Control in the Aerospace Industry, 1st ed.; Benavides, S., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2009; pp. 210–250. [Google Scholar]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical corrosion of titanium and titanium-based alloys. J. Prosthet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Effect of silicon on corrosion resistance of Ti-Si alloys. Mater. Sci. Eng. B 2011, 176, 79–86. [Google Scholar] [CrossRef]
- Escriva-Cerdán, C.; Blasco-Tamarit, E.; García-García, D.M.; Akid, R.; Walton, J. Effect of temperature on passive film formation of UNS N08031 Cr-Ni alloy in phosphoric acid contaminated with different aggressive anions. Electrochem. Acta 2013, 111, 552–561. [Google Scholar] [CrossRef]
- Liu, C.; Leyland, A.; Bi, Q.; Matthews, A. Corrosion resistance of multi-layerded plasma-assisted physical vapour deposition TiN and CrN coatings. Surf. Coat. Technol. 2001, 141, 164–173. [Google Scholar] [CrossRef]
- Balla, A.; Marcu, C.; Axante, D.; Borodi, G.; Lazar, D. Catalytic reduction of sulfuric acid to sulfur dioxide. Cent. Eur. J. Chem. 2012, 10, 1817–1823. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Dong, X.; Li, W.; Feng, W.; Xu, Y. Effect of solution and aging treatments on corrosion performance of laser solid formed Ti-6Al-4V alloy in a 3.5 wt. % NaCl solution. J. Mater. Res. Technol. 2020, 9, 1559–1568. [Google Scholar] [CrossRef]
- Ikumapayi, O.M.; Akinlabi, E.T. Efficacy of α-β grade titanium alloy powder (Ti-6Al-2Sn-2Zr-2Mo-2Cr-0.25Si). Mater. Res. Express 2019, 6, 2053–2063. [Google Scholar] [CrossRef]
- Nabhani, M.; Razavi, R.S.; Barekat, M. Corrosion study of laser cladded Ti-6Al-4V alloy in different corrosive environments. Eng. Fail. Anal. 2019, 97, 234–241. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G. Synergistic effects of fluoride and chloride on general corrosion behavior of AISI 316 stainless steel and pure titanium in H2SO4 solutions. Corros. Sci. 2018, 130, 203–217. [Google Scholar] [CrossRef]
- Engelkamp, B.; Fischer, B.; Schierbaum, K. Plasma Electrolytic Oxidation of Titanium in H2SO4-H3PO4 Mixtures. Coatings 2020, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Prando, D.; Nicolis, D.; Pedeferri, M.; Ormellese, M. Pitting corrosion on anodized titanium: Effect of halades. Mater. Corros. 2018, 69, 1–6. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Attarzadeh, F.R.; Vakili-Azghandi, M. Effect of Multi-pass Friction Stir processing on the Electrochemical and Corrosion Behavior of Pure Titanium in Strongly Acidic Solutions. Metall. Mater. Trans. A 2017, 48, 403–411. [Google Scholar] [CrossRef]
- Beck, T.R.; Blackburn, M.J. Stress corrosion cracking of titanium alloys. AIAA J. 1968, 6, 326–332. [Google Scholar] [CrossRef]
- Gao, K.W.; Chu, W.Y.; Gu, B.; Zhang, T.C.; Qiao, L.J. In-Situ Transmission Electron Microscopic Observation of Corrosion Enhanced Dislocation Emission and Crack Initiation of Stress Corrosion. Corrosion 2000, 56, 515–522. [Google Scholar] [CrossRef]
- Al-Mazeedi, H.A.A.; Cottis, R.A. A practical evolution of electrochemical noise parameters as indicators of corrosion type. Electrochim. Acta 2004, 49, 2787–2793. [Google Scholar] [CrossRef]
- Sanchez-Amaya, J.M.; Cottis, R.A.; Botana, F.J. Shot noise and statistical parameters to estimation of corrosion mechanisms. Corros. Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Eden, D.A.; Meng, Q.J.; Mendez, M.; Yunovich, M. Electrochemical Noise. In Uhlig’s Corrosion Handbook, 1st ed.; Revie, R.W., Ed.; John Wiley & Son: New York, NY, USA, 2011. [Google Scholar]
- Contreras, A.; Salazar, M.; Carmona, A.; Galván-Martínez, R. Electrochemical Noise for Detection of Stress Corrosion Cracking of Low Carbon Steel Exposed to Synthetic Soil Solution. Mater. Res. 2017, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini-Cervantes, M.J.; Almeraya-Calderon, F.; Borunda-Terrazas, A.; Bautista-Margulis, R.G.; Chacón-Nava, J.G.; Fajardo-San-Miguel, G.; Almaral-Sanchez, J.L.; Barrios-Durstewitz, C.; Martinez-Villafañe, A. Corrosion Resistance, Porosity and Strength of lended Portland Cement Mortar Containing Rice Husk Ash And Nano-SiO2. Int. J. Electrochem. Sci. 2013, 8, 10697–10710. [Google Scholar]
- Nagiub, A.; Mansfeld, F.; Sun, Z.; Hsu, C.H. Concerning trend removal in electrochemical noise measurements. Corros. Sci. 2001, 43, 341–352. [Google Scholar] [CrossRef]
- Arellano-Pérez, H.; Escobar-Jiménez, R.F.; Granados-Lieberman, F.; Gómez-Aguilar, J.F.; Uruchurtu-Chavarrín, J.; Alvarado-Martínez, V.M. Electrochemical noise signals evaluation to classify the type of corrosion using synchrosqueezing transform. J. Electroanal. Chem. 2019, 848, 113249. [Google Scholar] [CrossRef]
- Reid, S.; Bell, G.E.C.; Edgemon, G.L. The use of skewness, kurtosis and neural networks for determining mechanism from electrochemical noise data. In Corrosion/98; NACE International: San Diego, FL, USA, 22–27 March 1998; p. 176. [Google Scholar]
- Nazarnezhad-Bajastani, M.; Neshti, J.; Hossein Siadati, M. Determination of SS321 pitting stage in FeCl3 solution based on electrochemical noise measurement data using artificial neural network. J. Electroanal. Chem. 2019, 845, 31. [Google Scholar] [CrossRef]
- Cui, J.; Yu, D.; Long, Z.; Xi, B.; He, X.; Pei, Y. Application of electrochemical noise (EN) technology to evaluate the passivation performance of adsorption and film-forming type corrosion inhibitors. J. Electroanal. Chem. 2019, 855, 113584. [Google Scholar] [CrossRef]
- Li, J.; Du, C.W.; Liu, Z.Y.; Li, X.G.; Liu, M. Effect of microstructure on the corrosion resistance of 2205 duplex stainless steel. Part 2: Electrochemical noise analysis of corrosion behaviors of different microstructures based on wavelet transform. Constr. Build. Mater. 2018, 189, 1294–1302. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E, M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- Corral-Higuera, R.; Arredondo-Rea, P.; Neri-Flores, M.A.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Castorena-González, J.C.; Almeraya-Calderón, F. Chloride ion penetrability and Corrosion Behavior of Steel in Concrete with Sustainability Characteristics. Int. J. Electrochem. Sci. 2011, 6, 958–970. [Google Scholar]
- Moshrefi, R.; Mahjani, M.G.; Jafarian, M. Application of wavelet entropy in analysis of electrochemical noise for corrosion type identification. Electrochem. Commun. 2014, 48, 49–51. [Google Scholar] [CrossRef]
- Smith, M.T.; Macdonald, D.D. Wavelet Analysis of Electrochemical Noise Data. Corrosion 2009, 438–448. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Pitting mechanism on an austenite stainless steel nanocrystalline coating investigated by electrochemical noise and in-situ AFM analysis. Electrochim. Acta 2008, 54, 768–780. [Google Scholar] [CrossRef]
- Asfia, M.P.; Rezail, M.; Bahlakeh, K. Corrosion prevention of AISI 304 stainless steel in hydrochloric acid medium using garli extrac as a green corrosion inhibitor: Electrochemical and theorical studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Shahidi, M.; Hosseini, S.M.A.; Jafari, A.H. Comparison between ED and SDPS plots as the results of wavelet transform for analyzing electrochemical noise data. Electrochim Acta 2011, 56, 9986–9997. [Google Scholar] [CrossRef]
- Bajestani, M.Z.; Neshati, J.; Siadati, M.H. Development of Time-Frequency Analysis in Electrochemical Noise for Detection of Pitting Corrosion. Corrosion 2019, 75, 183–191. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Huang, J.C.; Chen, X.; Cao, L.; Mu, M. Affection of Cu content on the phase evolution during the dealloying of Ag-Cu alloys using electrochemical noise with Hilbert spectra analysis. Mater. Lett. 2016, 183, 165–169. [Google Scholar] [CrossRef]
- Homborg, A.M.; Morales, C.F.L.; Tinga, T.; de Wit, J.H.W.; Mol, J.M.C. Dection of microbiologically influenced corrosion by electrochemical transients. Electrochim. Acta 2014, 136, 223–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, E.; Xu, D.; Yang, Y.; Zhao, Y.; Zhang, Y.; Gu, T.; Yang, K.; Wang, F. Laboratory investigation of microbiologically influenced corrosion of 2205 duplex stainless steel by marine pseudomonas aeruginosa biofilm using electrochemical noise. Corrosion 2018, 143, 281–291. [Google Scholar] [CrossRef]
- Chui, C.K. An Introduction to Wavelets, 1st ed.; Chui, C.K., Ed.; Academic Press: San Diego, CA, USA, 1992; pp. 49–74. [Google Scholar]










| Titanium Alloy | Ti | Al | V | Zr | Mo | Sn | Fe |
|---|---|---|---|---|---|---|---|
| Ti CP2 | 99.94 ± 0.005 | - | - | - | - | - | 0.038 ± 0.005 |
| Ti-6Al-2Sn-4Zr-2Mo | 84.65 ± 0.19 | 6.75 ± 0.20 | - | 4.18 ± 0.01 | 1.99 ± 0.008 | 2.08 ± 0.01 | - |
| Ti-6Al-4V | 87.71 ± O.36 | 7.14 ± 0.37 | 4.03 ± 0.08 | - | - | - | 0.21 ± 0.01 |
| Corrosion Type | Potential | Current | ||
|---|---|---|---|---|
| Skewness | Kurtosis | Skewness | Kurtosis | |
| Uniform | <±1 | <3 | <±1 | <3 |
| Pitting | <−2 | >>3 | >±2 | >>3 |
| Transgranular (SCC) | 4 | 20 | −4 | 20 |
| Intergranular (SCC #1) | −6.6 | 18 to 114 | 1.5 to 3.2 | 6.4 to 15.6 |
| Intergranular (SCC #2) | −2 to −6 | 5 to 45 | 3 to 6 | 10 to 60 |
| Alloys | Electrolyte | Rn (Ω·cm2) | icorr (mA/cm2) | Skewness | Corrosion Type | Kurtosis | Corrosion Type |
|---|---|---|---|---|---|---|---|
| Ti CP2 | NaCl | 51503 | 5.05 × 10−4 | −0.8 | Uniform | 33 | pitting |
| H2SO4 | 11784 | 2.21 × 10−4 | −0.1 | Uniform | 5 | pitting | |
| Ti-6Al-2Sn-4Zr-2Mo | NaCl | 45123 | 5.76 × 10−4 | 3.5 | Localized | 29 | pitting |
| H2SO4 | 238943 | 1.09 × 10−4 | 1.1 | Localized | 8 | pitting | |
| Ti-6Al-4V | NaCl | 25643 | 1.01 × 10−3 | 2.6 | Localized | 35 | pitting |
| H2SO4 | 58564 | 4.44 × 10−4 | 1.2 | Localized | 5 | pitting |
| Corrosion Type | dB(V)·Decade−1 | dB(A)·Decade−1 | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| Uniform | 0 | −7 | 0 | −7 |
| Pitting | −20 | −25 | −7 | −14 |
| Passive | −15 | −25 | −1 | 1 |
| Alloys | Ψ0 (dBi) | Β (dB [A]) |
|---|---|---|
| NaCl | ||
| Ti CP2 | −122.29 | −12 |
| Ti-6Al-2Sn-4Zr-2Mo | −138.49 | −20 |
| Ti-6Al-4V | −121.13 | −15 |
| H2SO4 | ||
| Ti CP2 | −104.95 | −18 |
| Ti-6Al-2Sn-4Zr-2Mo | −134.93 | −8 |
| Ti-6Al-4V | −128.54 | −15 |
| Crystal (D) | Scale (s) | Scale (Hz) |
|---|---|---|
| 1 | 1–2 | 1–0.5 |
| 2 | 2–4 | 0.5–0.25 |
| 3 | 4–8 | 0.25–0.125 |
| 4 | 8–16 | 0.125–0.0625 |
| 5 | 16–32 | 0.0625–0.3125 |
| 6 | 32–64 | 0.03125–0.015625 |
| 7 | 64–128 | 0.015625–0.00781 |
| 8 | 128–256 | 0.00781–0.00390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. https://doi.org/10.3390/met11010105
Jáquez-Muñoz JM, Gaona-Tiburcio C, Cabral-Miramontes J, Nieves-Mendoza D, Maldonado-Bandala E, Olguín-Coca J, López-Léon LD, Flores-De los Rios JP, Almeraya-Calderón F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals. 2021; 11(1):105. https://doi.org/10.3390/met11010105
Chicago/Turabian StyleJáquez-Muñoz, Jesús Manuel, Citlalli Gaona-Tiburcio, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, Erick Maldonado-Bandala, Javier Olguín-Coca, Luis Daimir López-Léon, Juan Pablo Flores-De los Rios, and Facundo Almeraya-Calderón. 2021. "Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions" Metals 11, no. 1: 105. https://doi.org/10.3390/met11010105
APA StyleJáquez-Muñoz, J. M., Gaona-Tiburcio, C., Cabral-Miramontes, J., Nieves-Mendoza, D., Maldonado-Bandala, E., Olguín-Coca, J., López-Léon, L. D., Flores-De los Rios, J. P., & Almeraya-Calderón, F. (2021). Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals, 11(1), 105. https://doi.org/10.3390/met11010105