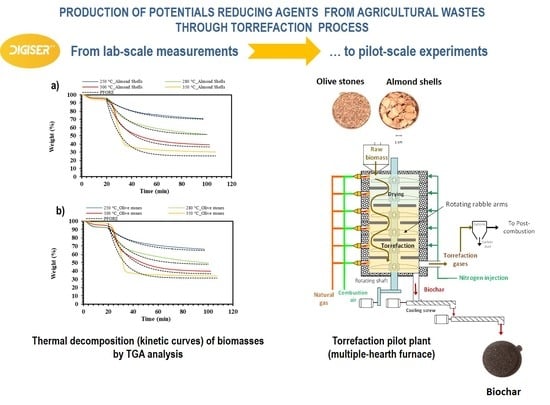
Upscaling Severe Torrefaction of Agricultural Residues to Produce Sustainable Reducing Agents for Non-Ferrous Metallurgy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Analysis
2.2. Torrefaction at Laboratory Scale
2.3. Torrefaction at Pilot Scale
2.4. Characterization of the Materials
2.4.1. Thermal Gravimetric Analyses (TGA)
2.4.2. Elemental Analyses
2.4.3. Calorimetric Analyses
2.4.4. SEM-EDX Analyses
3. Results and Discussion
3.1. Thermal Gravimetric Analyses (TGA)
- Stage 1: Corresponds to the increase in temperature from room temperature until around 200 °C. During this stage, mainly the loss of volatiles and water occurs, and the mass loss is similar for all temperatures and biomasses, reaching a maximum of 7 %w.
- Stage 2: Corresponds to the increase in temperature from around 200 °C until the torrefaction temperature. There is significant mass loss during this stage; the higher the temperature, the higher the mass loss. At 350 °C, the total mass loss reaches almost 70 %w for both biomasses. Significant differences among temperatures can be observed, reaching up to 20 %w. The higher the temperature, the more significant this mass decrease and the longer this stage is.
- Stage 3: Corresponds to the decrease in temperature from the torrefaction temperature to the room temperature. During this stage, there is no significant mass loss, being lower than 5 %w for all temperatures and biomasses. The higher the temperature, the later this stage takes place.
3.2. Torrefaction at Laboratory Scale
3.3. Characterization of the Materials
3.4. SEM-EDX Analyses
3.5. Torrefaction at Pilot Scale
4. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
Abbreviations
| HHV | higher heating value |
| PFORE | pseudo-first-order rate equation |
| db | dry basis |
References
- International Energy Agency. CO2 Emissions from Fuel Combustion 2018 Highlights. Available online: http://www.indiaenvironmentportal.org.in/files/file/CO2_Emissions_from_Fuel_Combustion_2018_Highlights.pdf (accessed on 24 October 2021).
- Alonso, E.R.; Dupont, C.; Heux, L.; Perez, D.D.S.; Commandre, J.M.; Gourdon, C. Study of solid chemical evolution in torrefaction of different biomasses through solid-state 13C cross-polarization/magic angle spinning NMR (nuclear magnetic resonance) and TGA (thermogravimetric analysis). Energy 2016, 97, 381–390. [Google Scholar] [CrossRef]
- Welfle, A. Balancing growing global bioenergy resource demands-Brazil’s biomass potential and the availability of resource for trade. Biomass Bioenergy 2017, 105, 83–95. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking—Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef] [Green Version]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Griessacher, T.; Antrekowitsch, J.; Steinlechner, S. Charcoal from agricultural residues as alternative reducing agent in metal recycling. Biomass Bioenergy 2012, 39, 139–146. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Jha, G.; Soren, S. Study on applicability of biomass in iron ore sintering process. Renew. Sustain. Energy Rev. 2017, 80, 399–407. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of biomass on temperatures of sintering and initial deformation of lignite ash. Fuel 2010, 89, 3063–3068. [Google Scholar] [CrossRef]
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A.T. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Ooi, T.C.; Aries, E.; Ewan, B.C.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Tognarelli, D. The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 2008, 21, 167–177. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction. Practical Design and Theory, 2nd ed.; Academic Press: Boston, MA, USA, 2013; pp. 87–145. [Google Scholar]
- Thevenon, F.; Marchand, M.; Grateau, M.; Demey, H.; Chatroux, A.; De Vincent, P.P.; De Ryck, A.; Melkior, T. Energy requirements to produce fine powders of raw and torrefied wood at pilot scale, and characterization of their flowability. Biomass Bioenergy 2021, 152, 106196. [Google Scholar] [CrossRef]
- Chiou, B.S.; Valenzuela-Medina, D.; Bilbao-Sainz, C.; Klamczynski, A.P.; Avena-Bustillos, R.J.; Milczarek, R.R.; Du, W.X.; Glenn, G.M.; Orts, W.J. Torrefaction of almond shells: Effects of torrefaction conditions on properties of solid and condensate products. Ind. Crops Prod. 2016, 86, 40–48. [Google Scholar] [CrossRef]
- Demey, H.; Melkior, T.; Chatroux, A.; Attar, K.; Thiery, S.; Miller, H.; Grateau, M.; Sastre, A.M.; Marchand, M. Evaluation of torrefied poplar-biomass as a low-cost sorbent for lead and terbium removal from aqueous solutions and energy co-generation. Chem. Eng. J. 2019, 361, 839–852. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. On the Experimental Evidence of Exothermicity in Wood and Biomass Pyrolysis. Energy Technol. 2017, 5, 19–29. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]

















| Property | Unit | Standard Procedure | Feedstock | |
|---|---|---|---|---|
| Almond Shells | Olive Stones | |||
| Moisture | %w | Internal method PA 254 | 14.8 | 8.3 |
| Ash | %wdb | Internal method PA209 or PA276 | 1.3 | 0.9 |
| Volatile matter | NF EN ISO 18123 | 79.1 | 79.2 | |
| Fixed C | Calculation/NF M03-006 | 19.6 | 19.9 | |
| C | NF EN ISO 16948 | 50.5 | 51.3 | |
| H | 5.95 | 5.99 | ||
| N | 0.20 | 0.19 | ||
| O | Calculation by difference | 42.0 | 41.6 | |
| S | Internal method PA 334 | 0.011 | 0.032 | |
| Cl | 0.009 | 0.020 | ||
| HHV | MJ/kg db | NF EN ISO 18125 | 19.84 | 20.30 |
| Experimental (TGA) | Fitting Model (PFORE) | ||||
|---|---|---|---|---|---|
| Biomass | Temperature (°C) | WTGA (%wdb) | KT (min−1) | Wp (%wdb) | r2 |
| Almond Shells | 250 | 24.9 ± 1.6 | 4.24 × 10−2 | 25.19 | 0.997 |
| 280 | 46.3 ± 0.4 | 5.40 × 10−2 | 45.00 | 0.986 | |
| 300 | 58.7 ± 1.3 | 6.39 × 10−2 | 58.31 | 0.985 | |
| 350 | 67.3 ± 1.1 | 9.01 × 10−2 | 67.27 | 0.941 | |
| Olive stones | 250 | 27.8 ± 0.9 | 4.51 × 10−2 | 27.82 | 0.997 |
| 280 | 45.7 ± 1.1 | 5.64 × 10−2 | 43.74 | 0.976 | |
| 300 | 57.9 ± 1.2 | 6.24 × 10−2 | 58.00 | 0.989 | |
| 350 | 65.0 ± 1.0 | 9.64 × 10−2 | 65.61 | 0.919 | |
| Thermal Gravimetric Analyses (TGA) | Laboratory Furnace | ||
|---|---|---|---|
| Biomass | Temperature (°C) | AWLTGA (%wdb) | AWLLab-Furnace (%wdb) |
| Almond shells | Raw | - | - |
| 250 | 24.94 | 29.80 | |
| 280 | 46.28 | 42.46 | |
| 300 | 58.66 | 49.97 | |
| 350 | 67.35 | 63.62 | |
| Olive stones | Raw | - | - |
| 250 | 27.79 | 25.63 | |
| 280 | 45.74 | 41.00 | |
| 300 | 57.91 | 50.14 | |
| 350 | 65.04 | 61.66 | |
| Elemental Analyses | Ash Content (815 °C) | Energy Content | Volatile Species | Fixed Carbon | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | Temp. (°C) | C (%) | H (%) | N (%) | S (%) | O (%) | Ash (%) | HHV * (MJ kg−1) | HHV ** (MJ kg−1) | VS (%) | FC (%) |
| Almond shells | 250 | 58.50 | 5.76 | 0.28 | 0.03 | 33.53 | 1.90 | 23.1 | 23.6 | 23.13 | 34.40 |
| 280 | 66.00 | 5.22 | 0.30 | 0.03 | 26.75 | 1.70 | 26.1 | 26.7 | 26.09 | 46.80 | |
| 300 | 69.20 | 5.20 | 0.31 | 0.02 | 23.67 | 1.60 | 27.5 | 28.4 | 27.52 | 53.10 | |
| 350 | 76.40 | 4.29 | 0.34 | 0.01 | 15.86 | 3.10 | 29.8 | 30.8 | 29.79 | 67.70 | |
| Olive stones | 250 | 59.30 | 5.57 | 0.22 | 0.03 | 33.68 | 1.20 | 23.9 | 23.8 | 23.94 | 34.40 |
| 280 | 66.20 | 5.46 | 0.24 | 0.03 | 26.87 | 1.20 | 26.8 | 27.1 | 26.83 | 46.00 | |
| 300 | 71.60 | 5.11 | 0.29 | 0.01 | 21.59 | 1.40 | 29.0 | 29.5 | 29.04 | 55.10 | |
| 350 | 76.40 | 4.32 | 0.33 | 0.02 | 16.93 | 2.00 | 30.2 | 30.9 | 30.28 | 68.30 | |
| Property | Unit | Feedstock | ||
|---|---|---|---|---|
| Almond Shells | Olive Stones | |||
| Initial moisture | %w | 14.8 | 8.3 | |
| Mass flow | Input | kg/h | 76.7 | 97.1 |
| kg db/h | 65.3 | 89.0 | ||
| Output | kg db/h | 23.9 | 34.5 | |
| Mass loss | %w | 69 ± 5% | 64 ± 4% | |
| %wdb | 63 ± 5% | 61 ± 4% | ||
| Bulk density of bed | - | 0.33 | 0.76 | |
| Porosity of bed | 0.72 | 0.50 | ||
| Characteristic | Unit | Standard Procedure | Feedstock | |
|---|---|---|---|---|
| Almond Shells | Olive Stones | |||
| Moisture | %w | Internal method PA 254 | <0.1 | <0.1 |
| Ash | %wdb | Internal method PA209 or PA276 | 2.5 | 2.1 |
| Volatile matter | NF EN ISO 18123 | 33.6 | 31.9 | |
| Fixed C | Calculation/NF M03-006 | 63.9 | 66.0 | |
| C | NF EN ISO 16948 | 74.3 | 76.3 | |
| H | 4.56 | 4.43 | ||
| N | 0.33 | 0.36 | ||
| O | Calculation by difference | 17.7 | 16.5 | |
| S | Internal method PA 334 | 0.016 | 0.015 | |
| Cl | <0.007 | 0.008 | ||
| HHV | MJ/kg db | NF EN ISO 18125 | 29.41 | 29.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demey, H.; Rodriguez-Alonso, E.; Lacombe, E.; Grateau, M.; Jaricot, N.; Chatroux, A.; Thiery, S.; Marchand, M.; Melkior, T. Upscaling Severe Torrefaction of Agricultural Residues to Produce Sustainable Reducing Agents for Non-Ferrous Metallurgy. Metals 2021, 11, 1905. https://doi.org/10.3390/met11121905
Demey H, Rodriguez-Alonso E, Lacombe E, Grateau M, Jaricot N, Chatroux A, Thiery S, Marchand M, Melkior T. Upscaling Severe Torrefaction of Agricultural Residues to Produce Sustainable Reducing Agents for Non-Ferrous Metallurgy. Metals. 2021; 11(12):1905. https://doi.org/10.3390/met11121905
Chicago/Turabian StyleDemey, Hary, Elvira Rodriguez-Alonso, Elie Lacombe, Maguelone Grateau, Nicolas Jaricot, André Chatroux, Sebastien Thiery, Muriel Marchand, and Thierry Melkior. 2021. "Upscaling Severe Torrefaction of Agricultural Residues to Produce Sustainable Reducing Agents for Non-Ferrous Metallurgy" Metals 11, no. 12: 1905. https://doi.org/10.3390/met11121905
APA StyleDemey, H., Rodriguez-Alonso, E., Lacombe, E., Grateau, M., Jaricot, N., Chatroux, A., Thiery, S., Marchand, M., & Melkior, T. (2021). Upscaling Severe Torrefaction of Agricultural Residues to Produce Sustainable Reducing Agents for Non-Ferrous Metallurgy. Metals, 11(12), 1905. https://doi.org/10.3390/met11121905